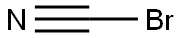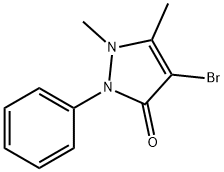
4-BROMOANTIPYRINE synthesis
- Product Name:4-BROMOANTIPYRINE
- CAS Number:5426-65-3
- Molecular formula:C11H11BrN2O
- Molecular Weight:267.12
60-80-0
468 suppliers
$10.00/5g

5426-65-3
36 suppliers
$59.00/5g
Yield:5426-65-3 90%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 20; for 0.5 h;
Steps:
4-Bromo-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one (26)
To a 100 mL flask antipyrine (0.94 g, 5 mmol) was dissolved in 10 mL of dichloromethane and allowed to stir in open atmosphere at 0 °C. Then N-bromosuccinimide [NBS] (0.88 g, 5mmol) was added portionwise to the solution over 30 min. After completed addition, the reaction was stirred at room temperature for 30 min. After the reaction time was over, it was quenched with saturated aqueous K2CO3 solution (30 mL). The reaction mixture was extracted with ethyl acetate. The organic portion was washed with water (2 × 100 mL) and dried over anhydrous Na2SO4. The organic portion was evaporated under vacuum to get the crude mixture, which was purified by silica column chromatography to give 26 in 90% yield (1.20 g). 1H NMR (400 MHz, CDCl3) δ (ppm) 7.45 - 7.33 (m, 2H), 7.30 - 7.27 (m, 2H), 7.25 - 7.18 (m, 1H), 3.01 (s, 3H), 2.22 (s, 3H). 13C NMR (75 MHz, CDCl3) δ (ppm) 162.1, 154.0, 129.4, 129.2, 127.0, 124.1, 90.5, 36.4, 12.5.
References:
Bera, Jitendra K.;Doucet, Henri;Sasmal, Arpan;Soulé, Jean-Fran?ois [Tetrahedron Letters,2020] Location in patent:supporting information
56-23-5
6 suppliers
$25.00/10ml
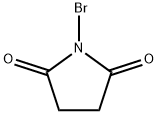
128-08-5
829 suppliers
$5.00/5G

83-10-3
54 suppliers
inquiry

5426-65-3
36 suppliers
$59.00/5g