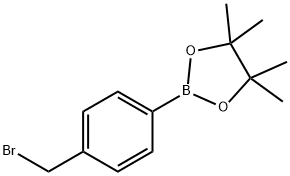
4-(Bromomethyl)benzeneboronic acid pinacol ester synthesis
- Product Name:4-(Bromomethyl)benzeneboronic acid pinacol ester
- CAS Number:138500-85-3
- Molecular formula:C13H18BBrO2
- Molecular Weight:297
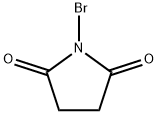
128-08-5
831 suppliers
$5.00/5 g
195062-57-8
152 suppliers
$5.00/250mg

138500-85-3
253 suppliers
$8.00/1g
Yield:138500-85-3 76.1%
Reaction Conditions:
with 2,2'-azobis(isobutyronitrile) in tetrachloromethane for 14 h;Reflux;
Steps:
4
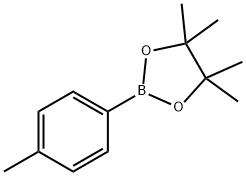
P-toluene boronic acid pinacol ester (10.91 g, 50.0 mmol),Bromosuccinimide (NBS, 9.91 g, 55.0 mmol),Azobisisobutyronitrile (AIBN, 0.44 g, 2.0 mmol) was dissolved in dry carbon tetrachloride (CCl4, 200 mL).It was stirred at reflux for 14 hours, filtered, the solvent was removed by rotary evaporation, and the ethyl acetate (200 mL) was dissolved.After washing once with distilled water (100 mL) and saturated aqueous sodium chloride solution (100 mL), it was dried over anhydrous sodium sulfate.Spin-steaming, finally using petroleum ether as eluent to cross the column,4-Bromomethylphenylboronic acid pinacol ester (11.30 g, yield 76.1%) was isolated.
References:
Nankai University;Li Changhua;Chen Haoliang CN107417714, 2017, A Location in patent:Paragraph 0056; 0057; 0061
302348-51-2
129 suppliers
$5.00/250mg

138500-85-3
253 suppliers
$8.00/1g

195062-57-8
152 suppliers
$5.00/250mg

138500-85-3
253 suppliers
$8.00/1g
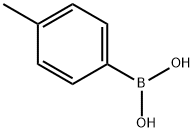
5720-05-8
427 suppliers
$5.00/1g

138500-85-3
253 suppliers
$8.00/1g
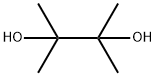
76-09-5
376 suppliers
$8.19/250mg

138500-85-3
253 suppliers
$8.00/1g