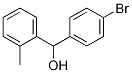
(4-BroMophenyl)(2-Methylphenyl)Methanol synthesis
- Product Name:(4-BroMophenyl)(2-Methylphenyl)Methanol
- CAS Number:944695-76-5
- Molecular formula:C14H13BrO
- Molecular Weight:277.1564
7294-50-0
1 suppliers
inquiry
1122-91-4
693 suppliers
$9.00/10g

944695-76-5
14 suppliers
$55.00/100mg
Yield:944695-76-5 91%
Reaction Conditions:
with C42H62ClO3PPd;sodium phosphate in toluene at 50; for 24 h;
Steps:
General procedure for palladacycle 1-catalyzed addition reactions of arylboroxines with aldehydes
General procedure: To a vial containing aldehyde (0.25 mmol), arylboroxine (0.167 mmol), K3PO4 (0.75 mmol) and palladacycle 1 (0.00125-0.0000125 mmol) (palladacycle 1 was added in a toluene solution: 1 mg/mL solution of palladacycle 1 in toluene was freshly prepared and 1-40 μL of this solution was added to the reaction system by microsyringes) was added toluene (1 mL). After the mixture was stirred at 50-90 oC for 20-60 hours, the reaction was quenched by adding a small amount of water. Column chromatography on silica gel with ethyl acetate/hexane (v/v=1:10) afforded the alcohols.
References:
Liao, Yuan-Xi;Xing, Chun-Hui;Israel, Matthew;Hu, Qiao-Sheng [Tetrahedron Letters,2011,vol. 52,# 26,p. 3324 - 3328] Location in patent:supporting information; experimental part
932-31-0
103 suppliers
$45.00/1g

1122-91-4
693 suppliers
$9.00/10g

944695-76-5
14 suppliers
$55.00/100mg
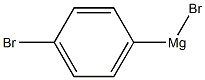
18620-02-5
17 suppliers
inquiry
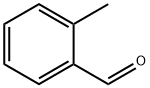
529-20-4
341 suppliers
$5.00/5g

944695-76-5
14 suppliers
$55.00/100mg