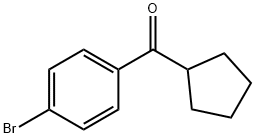
(4-bromophenyl)(cyclopentyl)methanone synthesis
- Product Name:(4-bromophenyl)(cyclopentyl)methanone
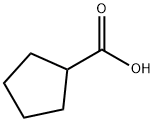
- CAS Number:2204-97-9
- Molecular formula:C12H13BrO
- Molecular Weight:253.13

108-86-1
496 suppliers
$10.00/5g

4524-93-0
136 suppliers
$13.43/1gm:

2204-97-9
14 suppliers
$417.00/1g
Yield:2204-97-9 73%
Reaction Conditions:
with aluminum (III) chloride at 20; for 15 h;Inert atmosphere;
Steps:
49.2 Step 2: (4-Bromophenyl)(cyclopentyl)methanone
[00563] To a solution of Aids (10.0 g, 75.4 mmol) and bromobenzene (17.76 g, 113.13 mmol) was added cyclopentanecarbonyl chloride (10.0 g, 75.42 mmol). The mixture was stirred at room temperature for 15 hours under a nitrogen atmosphere. The reaction mixture was concentrated and purified by silica gel column chromatography (0 - 5% EtOAc in petroleum ether) to afford the title compound (9.5 g, 73%) as a colorless oil. 3H NMR (400 MHz, CDCb): d 7.83 (d, J= 8.8 Hz, 2H), 7.58 (d, J= 8.8 Hz, 2H), 3.68 - 3.60 (m, 1H), 1.91 - 1.88 (m, 411 K 1.70 - 1.64 (m, 4H).
References:
WO2021/108483,2021,A1 Location in patent:Paragraph 00559; 00562-00563
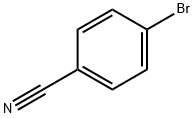
623-00-7
469 suppliers
$6.00/10g

33240-34-5
182 suppliers
$92.00/100g

2204-97-9
14 suppliers
$417.00/1g
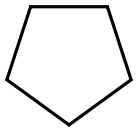
287-92-3
290 suppliers
$22.00/25mL
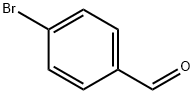
1122-91-4
672 suppliers
$9.00/10g

2204-97-9
14 suppliers
$417.00/1g
3400-45-1
360 suppliers
$10.00/5g

2204-97-9
14 suppliers
$417.00/1g