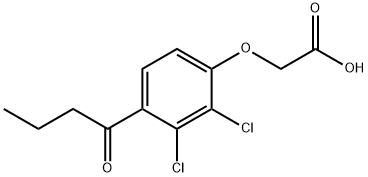
4-BUTYRYL-2,3-DICHLOROPHENOXYACETICACID synthesis
- Product Name:4-BUTYRYL-2,3-DICHLOROPHENOXYACETICACID
- CAS Number:1217-67-0
- Molecular formula:C12H12Cl2O4
- Molecular Weight:291.13
Yield:1217-67-0 95%
Reaction Conditions:
Stage #1: 2',3'-dichloro-4'-hydroxybutyrophenonewith potassium carbonate;potassium iodide in acetone; for 0.5 h;
Stage #2: ethyl bromoacetate in acetone at 60; for 4 h;
Stage #3: with potassium hydroxide in ethanol;water at 20; for 2 h;
Steps:
3 Embodiment 3:2 - [2, 3 - dichloro -4 - d acyl phenoxy] acetic acid (4) preparation of
The 2, 3 - dichloro -4 - d acyl phenol (26.0 g, 111.5 mmol) is placed in the reaction bottle, adds the acetone 120 ml dissolved, sequentially adding potassium carbonate (16.9 g, 122.7 mmol), potassium iodide (3.7 g, 22.3 mmol), magnetic stirring 0.5 h, instillment bromine ethyl acetate (20.5 g, 122.7 mmol), heating to 60 °C reaction 4 h, cooled to the room temperature, evaporate acetone, adding water and ethyl acetate, separating the aqueous phase, then washing the organic phase two, reducing pressure and solvent to obtain oil object, then adding ethanol 80 ml dissolved, adding water 80 ml, potassium hydroxide (12.5 g, 223.0 mmol), the reaction at room temperature 2 h, reducing pressure and ethanol, drops of concentrated hydrochloric acid to pH=1, a large number of white solid precipitated, filtering, drying to obtain 2 - [2, 3 - dichloro -4 - d acyl phenoxy] acetic acid.2 - [2, 3 - dichloro -4 - d acyl phenoxy] acetic acid: white solid; 30.8 g, yield 95.0%;
References:
CN106916116,2017,A Location in patent:Paragraph 0069; 0070; 0071
58-54-8
154 suppliers
$35.00/1g

1217-67-0
25 suppliers
inquiry
576-24-9
240 suppliers
$14.00/1g

1217-67-0
25 suppliers
inquiry
1984-59-4
204 suppliers
$20.00/25g

1217-67-0
25 suppliers
inquiry

