
4-CARBOXY-2,6-DIFLUOROBENZALDEHYDE synthesis
- Product Name:4-CARBOXY-2,6-DIFLUOROBENZALDEHYDE
- CAS Number:736990-88-8
- Molecular formula:C8H4F2O3
- Molecular Weight:186.11
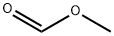
107-31-3
455-40-3

736990-88-8
Example 1. Preparation of 3,5-difluoro-4-formylbenzoic acid. To a solution of 3,5-difluorobenzoic acid (291 g, 1.84 mol) in 2-methyltetrahydrofuran (4.35 L) was added N,N,N',N'-tetramethylethylenediamine (TMEDA) (604 mL, 4.03 mol) at room temperature. The resulting solution was cooled to -78 °C. Subsequently, n-butyllithium (n-BuLi) (2.5 M hexane solution) (1.77 L, 4.43 mol) was added slowly and dropwise, ensuring that the temperature of the mixture was maintained below -65 °C during the process. After dropwise addition, the reaction mixture was continued to be stirred at -78 °C for 1.5 hours. Next, anhydrous methyl formate (MeOCHO) (239 mL, 3.88 mol) was added dropwise at a rate that kept the temperature below -65 °C. The reaction solution was gradually warmed to room temperature and stirred continuously at this temperature for 18 hours. Upon completion of the reaction, the mixture was cooled to 0-5 °C and the excess base was quenched with 6 M aqueous hydrochloric acid (2.2 L, 13.2 mol). The organic and aqueous phases were separated and the aqueous phase was extracted three times with 2-methyltetrahydrofuran (3 x 500 mL). The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure. The residue was dissolved in ethyl acetate (350 mL), heated to reflux to dissolve and cooled to room temperature. Ethane (480 mL) was added and the mixture was further cooled to -15 °C. The precipitated solid was collected by filtration, washed with hexane and dried under vacuum to afford the target product 3,5-difluoro-4-formylbenzoic acid (122 g, 35% yield) as a white solid. The product was characterized by 1H NMR (300 MHz, DMSO-d6): δ 7.63-7.70 (m, 2H), 10.23 (s, 1H); mass spectrum (ESI) m/z: 187.17 [M+H]+.
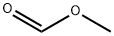
107-31-3
356 suppliers
$16.00/25mL

455-40-3
319 suppliers
$10.00/5g

736990-88-8
77 suppliers
$36.00/100mg
Yield: 36%
Reaction Conditions:
Stage #1:3,5-difluorobenzoic acid with n-butyllithium;N,N,N,N,-tetramethylethylenediamine in 2-methyltetrahydrofuran;hexane at -78 - -65; for 1.5 h;
Stage #2:Methyl formate in 2-methyltetrahydrofuran;hexane at -78 - 20; for 18.75 h;
Stage #3: with hydrogenchloride in water at 0 - 5;
Steps:
Intermediate compound 32: 3,5-difluoro-4-formylbenzoic acid
To a solution of 3,5-difluorobenzoic acid (20 g, 126.5 mmol) in 2- methyltetrahydrofuran (225 ml_) Ν,Ν,Ν',Ν'-tetramethylethylenediamine (TMEDA) (2.2 eq., 278.3 mmol, 43 ml_) was added at room temperature. The resulting solution was cooled to -785C and "Butyllithium ("BuLi 2.5 M in hexane) (2.5 eq., 316.2 mmol, 126.5 ml_) was added dropwise, during which the temperature of the mixture remained at less than -655C. The mixture was then stirred at -785C for 1 .5 h. Anhydrous methyl formate (2.1 eq., 265.6 mmol, 16.4 ml_) was added dropwise at a rate that allowed the temperature to be maintained at less than - 655C. The resulting solution was allowed to stir at -785C for 45 min, then the bath was removed and the reaction mixture was allowed to warm to room temperature while being stirred for 18h. The mixture was then cooled to 0-55C and excess base was quenched with 6 M aqueous HCI (7 eq., 885.5 mmol, 140 ml_). The phases were then separated, and the aqueous layer was extracted 3 times with ethyl acetate. The combined organic phases were washed with 1 N HCI solution, saturated brine, dried over anhydrous sodium sulphate, filtered and concentrated under vacuum. The residue was triturated in the minimum amount of ethyl acetate. A white solid precipitated was collected by filtration, rinsed with hexanes and dried under vacuum to form the title compound (8.48g, Yield: 36%) as a solid. 1 H NMR (400 MHz, CDCI3) δ 10.41 (1 H, s), 7.72 (2H, d, J = 8.4 Hz). 1 H NMR (400 MHz, DMSO-d6) δ 13.95 (1 H,s), 10.22 (1 H, s), 7.65 (2H, d, J
References:
ALMIRALL, S.A.;DRACONIS PHARMA, S.L.;LABORATORIOS DEL DR. ESTEVE, S.A.;AGUILAR, Nuria;FERNANDEZ, Joan, Carles;TERRICABRAS, Emma;CARCELLER GONZALEZ, Elena;GARCIA GARCIA, Francisco, Javier;SALAS SOLANA, Jordi WO2013/149996, 2013, A1 Location in patent:Page/Page column 56; 57