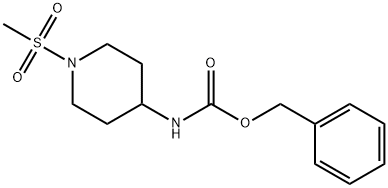
4-(Cbz-aMino)-1-(Methylsulfonyl)piperidine synthesis
- Product Name:4-(Cbz-aMino)-1-(Methylsulfonyl)piperidine
- CAS Number:402927-96-2
- Molecular formula:C14H20N2O4S
- Molecular Weight:312.38
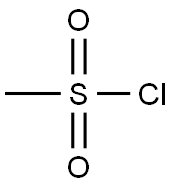
124-63-0
6 suppliers
$10.00/5g
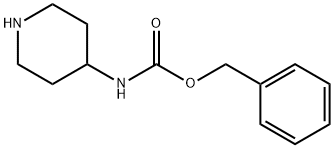
182223-54-7
122 suppliers
$63.00/1g

402927-96-2
19 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 3 h;
Steps:
4.3 Step 3; Benzyl 1-(methylsulfonyl)piperidin-4-ylcarbamate
The protected piperidine 13 (10 g, 42.7 mmol) and triethylamine (12 mL, 86.7 mmol) was dissolved in 500 mL of methylene chloride at room temperature. Methane sulfo-nylchloride (4.3 mL, 55.5 mmol) in 20 mL of methylene chloride was added dropwise via addition funnel. After addition was complete, the reaction mixture was stirred at room temperature for 3 hours. The solvent and volatiles were removed under reduced pressure. Ethyl acetate (500 mL) and an aqueous solution of hydrochloric acid (0.5M, 350 mL) was added. The reaction was partitioned between the two phases and the aqueous layer was removed. The organic layer was washed again with an aqueous solution of hydrochloric acid (0.5M, 2x, 100 mL) and then with saturated aqueous sodium bicarbonate solution (3x, 100 mL). The reaction solvent was then dried (brine, MgSO4) and evaporated at reduced pressure to provide 9.2 g of the methane sulfonamide 14 (MP=148.6-152.8 C.).
References:
US2003/232847,2003,A1 Location in patent:Page 14-15