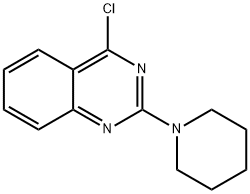
4-Chloro-2-(1-piperidinyl)quinazoline, 97% synthesis
- Product Name:4-Chloro-2-(1-piperidinyl)quinazoline, 97%
- CAS Number:134962-82-6
- Molecular formula:C13H14ClN3
- Molecular Weight:247.72
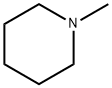
626-67-5
215 suppliers
$15.00/25g
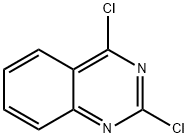
607-68-1
310 suppliers
$10.00/1g

134962-82-6
10 suppliers
$517.00/1g
Yield:134962-82-6 72%
Reaction Conditions:
in 1,4-dioxane at 150; for 0.0833333 h;Microwave irradiation;
Steps:
1.3
2,4-Dichloroquinazoline (1.5 g, 7.5 mmol) was dissolved in 1,4-dioxane (15 mL). N-Methylpiperidine (961 L, 7.9 mmol) was added and the mixture was heated to 150° C. for 5 min under microwave irradiation. The mixture was diluted with EtOAc and washed with saturated aqueous NaHCO3 and brine (×2). The organic layer was dried (MgSO4), filtered and concentrated in vacuo. The crude product was purified by column chromatography (5% EtOAc-petrol) to afford the title compound as a yellow oil that solidified on evaporation from petrol to a yellow solid (1.33 g, 72%).1H NMR (DMSO): 7.95-7.91 (1H, m), 7.77 (1H, ddd, J 8.5, 7.0 and 1.4), 7.54-7.49 (1H, m), 7.32 (1H, ddd, J 8.1, 7.0 and 1.1), 3.84-3.79 (4H, m) and 1.69-1.51 (6H, m).
References:
US2010/317607,2010,A1 Location in patent:Page/Page column 24

626-67-5
215 suppliers
$15.00/25g
86-96-4
246 suppliers
$22.00/25g

134962-82-6
10 suppliers
$517.00/1g

14446-67-4
38 suppliers
$45.00/10mg

86-96-4
246 suppliers
$22.00/25g

134962-82-6
10 suppliers
$517.00/1g

86-96-4
246 suppliers
$22.00/25g
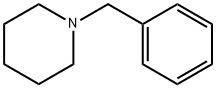
2905-56-8
112 suppliers
$20.00/1g

134962-82-6
10 suppliers
$517.00/1g

14446-67-4
38 suppliers
$45.00/10mg

607-68-1
310 suppliers
$10.00/1g

134962-82-6
10 suppliers
$517.00/1g