
4-CHLORO-2,3-DIHYDRO-1H-INDEN-1-OL synthesis
- Product Name:4-CHLORO-2,3-DIHYDRO-1H-INDEN-1-OL
- CAS Number:3199-71-1
- Molecular formula:C9H9ClO
- Molecular Weight:168.62
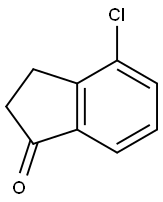
15115-59-0
147 suppliers
$8.00/250mg

3199-71-1
12 suppliers
inquiry
Yield:3199-71-1 83%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 20 - 25; for 2 h;
Steps:
84.1 4-Chloro-2,3-dihydro- 1H-inden- 1 -ol
NaBH4 (227 mg, 6 mmol) was added to the solution of 4-Chloro-2,3-dihydro-1H- inden-1-one (500 mg, 3 mmol) in MeOH (5 mL) and the reaction mixture was stirred at RT for 2 h. After completion of reaction, the solvent was evaporated and the residue obtained was dissolved in EtOAc and was washed with water. Organic layer was collected and dried over Na2504 and evaporated to obtain the title compound (422 mg). Yield: 83 %; 1H NMR (CDC13, 300 MHz): 7.18-7.32 (m, 3H), 5.27-5.30 (m, 1H), 3.06-3.16 (m, 1H), 2.80-2.90 (m, 1H), 2.47-2.58 (m, 1H), 1.9 1-2.05 (m, 2H).
References:
WO2015/28960,2015,A1 Location in patent:Page/Page column 129
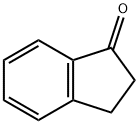
83-33-0
549 suppliers
$6.00/5g

3199-71-1
12 suppliers
inquiry
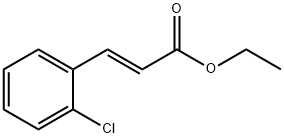
24393-51-9
12 suppliers
$45.00/50mg

3199-71-1
12 suppliers
inquiry

89-98-5
635 suppliers
$15.00/25g

3199-71-1
12 suppliers
inquiry
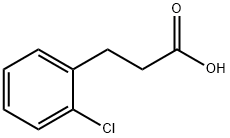
1643-28-3
154 suppliers
$8.00/1g

3199-71-1
12 suppliers
inquiry