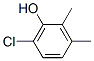
4-CHLORO-2,3-DIMETHYLPHENOL synthesis
- Product Name:4-CHLORO-2,3-DIMETHYLPHENOL
- CAS Number:1570-76-9
- Molecular formula:C8H9ClO
- Molecular Weight:156.61
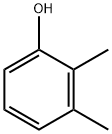
526-75-0
199 suppliers
$10.00/1g

1570-76-9
8 suppliers
inquiry
Yield:1570-76-9 84%
Reaction Conditions:
with N-chloro-succinimide;toluene-4-sulfonic acid in acetonitrile at 20; for 3 h;
Steps:
26.1; 27.1 Step 1: 4-chloro-2,3-dimethylphenol
To a solution of 2,3-dimethylphenol (204 mg, 1.67 mmol) in acetonitrile (16.7 mL) at room temperature was added / TsOH (635 mg, 3.34 mmol). Upon complete dissolution, this was followed by the addition of A-chl orosucci ni mi de (223 mg, 1.67 mmol) in a single portion. After 2 h, another aliquot of A-chlorosuccinimide (32 mg, 0.240 mmol) was added. The reaction mixture was stirred for 1 h more and then diluted with water and excess solid Na2SCb was added to quench excess reagent. The reaction mixture was diluted with EtOAc, and the aqueous layer was extracted three times with EtOAc. The organic layer was dried over sodium sulfate, filtered, and concentrated to obtain a crude white solid. This material was taken up in DCM and loaded onto a silica gel for purification by column chromatography eluting in Hex/DCM 0-70% and then Hex/EtOAc 0-100% to obtain 4-chloro-2,3-dimethylphenol (220 mg, 1.41 mmol, 84% yield). HPLC /R 0.85 min (analytical HPLC Method TS1). NMR (499 MHz, CHLOROFORM-d) d 7.06 (d, J= 8.5 Hz, 1H), 6.58 (d, =8.7 Hz, 1H), 4.70 (br s, 1H), 2.33 (s, 3H), 2.20 (s, 3H).
References:
WO2021/67657,2021,A1 Location in patent:Page/Page column 70-71
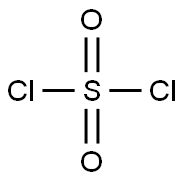
7791-25-5
255 suppliers
$15.00/0.5g

526-75-0
199 suppliers
$10.00/1g

64-19-7
1537 suppliers
$10.00/25ML

1570-76-9
8 suppliers
inquiry