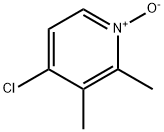
4-Chloro-2,3-dimethylpyridine 1-oxide synthesis
- Product Name:4-Chloro-2,3-dimethylpyridine 1-oxide
- CAS Number:59886-90-7
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
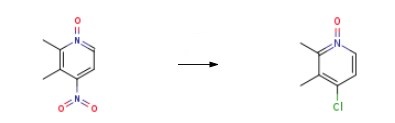
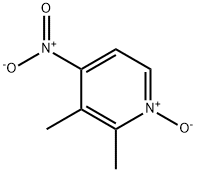
37699-43-7
368 suppliers
$10.00/1g

59886-90-7
249 suppliers
$25.00/250 mg
Yield:59886-90-7 100%
Reaction Conditions:
with acetyl chloride in ethanol at 65; for 5 h;
Steps:
26 The synthesis of X4-349-4
To a solution of X4-348-3 (10.0 g, 59.47 mmol) in ethanol (100.0 mL) was slowly added acetyl chloride (10.6 mL, 148.68 mmol). The mixture was stirred at 65 °C for 5 h. The solvent was removed under reduce pressure, diluted with water and DCM, aqueous NaOH (5 0%) was added dropwise to adjust pH to 7.5-8.5. The organic layer was washed with brine, dried over Na2SO4, filtered and concentrated in vacuum. The crude X4-349-4 (9.4 g, 100% yield) was used in next step without further purification. LCMS (Agilent LCMS 1200-6120, Column: Waters XBridge C18 (50 mm*4.6 mm*3.5 iim); Column Temperature: 40 °C; Flow Rate: 2.0 mL/min;Mobile Phase: from 90% [(total 10mM AcONH4) water/CH3CN = 900/100 (v/v)] and 10% [(total 10mM AcONH4) water/CH3CN = 100/900 (v/v)] to 10% [(total 10mM AcONH4) water/CH3CN = 900/100 (v/v)] and 90% [(total 10mM AcONH4) water/CH3CN = 100/900 (v/v)] in 1.6 mm, then under this condition for 2.4 mm, finally changed to 90% [(total the 10mM AcONH4) water/CH3CN = 900/100 (v/v)] and 10% [(total 10mM AcONH4) water/CH3CN = 100/900 (v/v)] in 0.1 mm and under this condition for 0.7 mm). Purity: 95.35%; Rt = 0.65 mm; MS Calcd.: 157.0; MS Found: 158.4[M+H]t
References:
X4 PHARMACEUTICALS, INC.;BOURQUE, Elyse Marie Josee;SKERLJ, Renato WO2017/223229, 2017, A1 Location in patent:Paragraph 00452; 00453
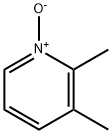
22710-07-2
141 suppliers
$16.00/1g

59886-90-7
249 suppliers
$25.00/250 mg

37699-43-7
368 suppliers
$10.00/1g

75-36-5
561 suppliers
$17.92/100G

59886-90-7
249 suppliers
$25.00/250 mg
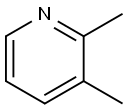
583-61-9
340 suppliers
$5.00/5g

59886-90-7
249 suppliers
$25.00/250 mg