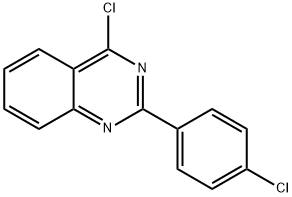
4-CHLORO-2-(4-CHLORO-PHENYL)-QUINAZOLINE synthesis
- Product Name:4-CHLORO-2-(4-CHLORO-PHENYL)-QUINAZOLINE
- CAS Number:59490-94-7
- Molecular formula:C14H8Cl2N2
- Molecular Weight:275.13
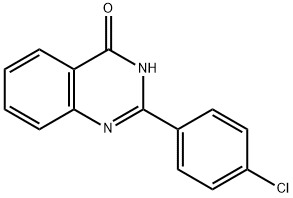
7455-77-8
22 suppliers
inquiry

59490-94-7
21 suppliers
inquiry
Yield:59490-94-7 88%
Reaction Conditions:
with thionyl chloride in N,N-dimethyl-formamide; for 2 h;Reflux;
Steps:
3.3. Oxidative aromatisation of 3a -3e in SOCl 2 -DMF mixture
General procedure: 4-Chloroquinazoline derivatives were prepared following a lit- erature method reported by Mphahlele and co-workers [16] . To a stirring suspension of 3a (1.00 g, 3.90 mmol) in thionyl chloride (30 mL) at room temperature, DMF (1 mL) was added dropwise. The mixture was refluxed for 2 h and allowed to cool to room tem- perature before quenching with cold water and was extracted with dichloromethane. The dichloromethane was dried over anhydrous sodium sulphate, filtered and evaporated under reduced pressure to yield compounds 4a -4d [14] and 4e [15] . The following prod- ucts were prepared accordingly. 4-Chloro-2-(4-chlorophenyl)quinazoline ( 4a ). A stirred mixture of 3a (1.00 g, 3.90 mmol) and DMF (1 mL) in thionyl chloride (30 mL) yielded 4a as a white solid (0.94 g, 88%); m.p. 143.2-145.4 °C; FT-IR(v max ) 758, 842, 954, 1152, 1234, 1336, 1519, 1537; 1 H-NMR(400 MHz, CDCl 3 , ppm) 7.47 (d, J = 8.8 Hz, 2 H), 7.60-7.56 (m, 1 H), 7.89-7.85 (m, 1 H), 8.05 (d, J = 8 Hz, 1H), 8.23 (d, J = 8 Hz, 1 H), 8.56 (d, J = 8.8 Hz, 2 H); 13 C-NMR (100 MHz, CDCl 3 , ppm) 122.4, 125.8, 128.4, 128.9, 130.1, 135.0, 137.4, 143.7, 151.7, 159, 162.6. [14] .
References:
Dilebo, Kabelo B.;Gumede, Njabulo J.;Mampa, Richard M.;Mangokoana, Dikgale;Matsebatlela, Thabe M.;Moraone, Ngaoko R.;Nxumalo, Winston;Omondi, Bernard [Journal of Molecular Structure,2021,vol. 1243]
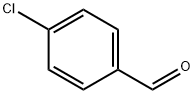
104-88-1
610 suppliers
$5.00/25g

59490-94-7
21 suppliers
inquiry
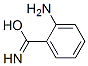
28144-70-9
6 suppliers
inquiry

59490-94-7
21 suppliers
inquiry

18600-52-7
8 suppliers
$106.00/250mg

59490-94-7
21 suppliers
inquiry
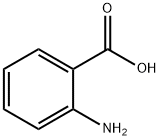
118-92-3
4 suppliers
$25.89/25g

59490-94-7
21 suppliers
inquiry