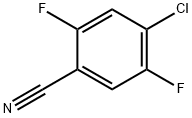
4-CHLORO-2,5-DIFLUOROBENZONITRILE synthesis
- Product Name:4-CHLORO-2,5-DIFLUOROBENZONITRILE
- CAS Number:135748-35-5
- Molecular formula:C7H2ClF2N
- Molecular Weight:173.55

508203-26-7
13 suppliers
inquiry

135748-35-5
124 suppliers
$14.00/250mg
Yield:135748-35-5 41.57 g
Reaction Conditions:
with trichlorophosphate at 80;
Steps:
5.5 4-Bromo-2,5-difluorobenzonitrile (9h)
General procedure: A mixture of 4-bromo-2,5-difluorobenzoic acid (11a, 53.28 g), thionyl chloride (165 mL) and DMF (0.87 mL) was stirred at 80 °C for 1.5 h, and cooled down to room temperature. The mixture was evaporated in vacuo, and the resulting residue was dissolved in chloroform (300 mL). To the solution was added dropwise 28 wt % aqueous ammonia (300 mL) at 5 °C, and the mixture was stirred at 5 °C for 0.5 h. The mixture was extracted with chloroform, and the organic layer was dried. The desiccant was removed by filtration and the filtrate was evaporated in vacuo to obtain a pale yellow solid. The mixture of the obtained solid and phosphoryl chloride (195 mL) was stirred at 80 °C for 2 h, and cooled down to room temperature. The mixture was evaporated in vacuo, and the resulting residue was treated with diethyl ether (500 mL) and ice-water (300 mL), then stirred at room temperature for 0.5 h. The mixture was extracted with diethyl ether and the organic layer was washed with saturated aqueous sodium hydrogen carbonate and brine, and then dried. The desiccant was removed by filtration and the filtrate was evaporated in vacuo to obtain 9h (41.57 g, 85%) as a pale yellow solid.
References:
Negoro, Kenji;Yonetoku, Yasuhiro;Misawa-Mukai, Hana;Hamaguchi, Wataru;Maruyama, Tatsuya;Yoshida, Shigeru;Takeuchi, Makoto;Ohta, Mitsuaki [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 17,p. 5235 - 5246]

132794-08-2
59 suppliers
$27.00/5g

135748-35-5
124 suppliers
$14.00/250mg
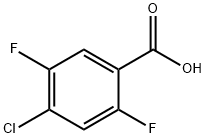
132794-07-1
168 suppliers
$5.00/1g

135748-35-5
124 suppliers
$14.00/250mg