
4-Chloro-2,6-diMethylquinazoline synthesis
- Product Name:4-Chloro-2,6-diMethylquinazoline
- CAS Number:1429782-21-7
- Molecular formula:C10H9ClN2
- Molecular Weight:192.64

18731-19-6
73 suppliers
$22.00/100mg

1429782-21-7
37 suppliers
inquiry
Yield:1429782-21-7 64.7%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate in toluene at 80; for 4 h;
Steps:
Synthesis of 4-chloro-6-methyl-2-methyl-quinazoline
Synthesis of 4-chloro-6-methyl-2-methyl-quinazoline 19 21A solution of the compound 19 (5 g, 32 mmol) in AC2O(25 mL) was heated at relux for 12 hours and the mixture was concentrated togive the solid. The solid was dissolved in amixture of the EtOH(50 ml) and the NH3.H2O(50 ml). The mixture wasstirred at 80°C for 5 hours, when TLC (EtOAc/PE = 1 :1 ,) showed the startingmaterial was consumed, and a new spot wasappeared, the reaction mixture was cooled to room temperature, filtered and thefilter cake was collected to give the compound 21 (3.5 g, yield: 60.9%).To the solution of the compound 21 (7 g, 40 mmol) in toluene (70 mL) wasadded the DIPEA (10.3 g, 80 mmol) and the POCI3 (10.4 g, 64 mmol). The mixture was stirred at 80°C for 4 hours, whenTLC (PE/EtOAc = 3:1 ) showed the starting material compound 21 was consumed,and a new spot was appeared, the reactionmixture was concentrated to give the crude compound which was purified bycolumn chromatography on silica gel (PE/EtOAc =20:1 ) to give the desiredcompound 4-chloro-6-methyl-2-methyl-quinazoline (5 g, yield: 64.7%) as a white solid.1H NMR (CDCI3 400 MHz): 5=7.94 (s,1 H),7.84 (d, J = 8.8 Hz, 1H), 7.72 (m, J = 10.8 Hz 1 H), 2.81 (s, 3H), 2.55 (s, 3H).
References:
WO2013/50527,2013,A1 Location in patent:Page/Page column 58; 59
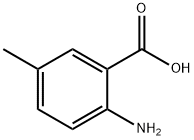
2941-78-8
476 suppliers
$5.00/10g

1429782-21-7
37 suppliers
inquiry