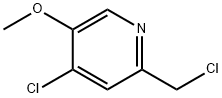
4-chloro-2-chloromethyl-5-methoxy-pyridine synthesis
- Product Name:4-chloro-2-chloromethyl-5-methoxy-pyridine
- CAS Number:28104-31-6
- Molecular formula:C7H7Cl2NO
- Molecular Weight:192.04
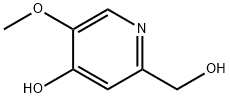
62885-42-1
14 suppliers
$133.00/5G

28104-31-6
16 suppliers
$526.00/250mg
Yield:28104-31-6 35 %
Reaction Conditions:
with trichlorophosphate in acetonitrile at 70;
Steps:
Intermediate 4: 4-Chloro-2-(chloromethyl)-5-methoxypyridine
To a solution of 2-(hydroxymethyl)-5-methoxypyridin-4-ol (50 mg, 0.32 mmol) in MeCN (2 mL) was added phosphorus oxychloride (0.090 mL, 0.98 mmol), and the mixture was heated at 70 °C for 22 h. After this time, additional phosphorus oxychloride (0.090 mL, 0.98 mmol) was added, and heating was continued for 20 h. A third portion of phosphorus oxychloride (0.090 mL, 0.98 mmol) was then added, and heating continued for 20 h. After this time, the volatiles were removed under reduced pressure, and the residue obtained was diluted with EtOAc (20 mL) and neutralized with sat. sodium bicarbonate solution. The layers were separated, and the aqueous layer was extracted with EtOAc (10 mL). The combined organic layers were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue obtained was purified by FCC (silica, 0-10% MeOH in DCM) to afford the title compound (22 mg, 35%).1H NMR (300 MHz, CDCl3) 8.22 (s, 1H), 7.49 (s, 1H), 4.61 (s, 2H), 4.01 (s, 3H). MS (ES+) (M+H)+ 192.
References:
WO2022/216947,2022,A1 Location in patent:Page/Page column 57
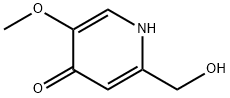
6323-21-3
21 suppliers
$65.00/100mg

28104-31-6
16 suppliers
$526.00/250mg

501-30-4
615 suppliers
$5.00/25mg

28104-31-6
16 suppliers
$526.00/250mg
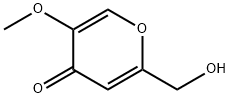
6269-25-6
38 suppliers
$244.00/500mg

28104-31-6
16 suppliers
$526.00/250mg