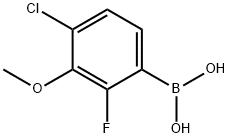
4-Chloro-2-fluoro-3-methoxyphenylboronic acid synthesis
- Product Name:4-Chloro-2-fluoro-3-methoxyphenylboronic acid
- CAS Number:944129-07-1
- Molecular formula:C7H7BClFO3
- Molecular Weight:204.39
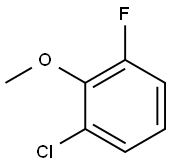
53145-38-3
112 suppliers
$8.00/1g

944129-07-1
81 suppliers
$45.00/25mg
Yield:944129-07-1 93%
Reaction Conditions:
Stage #1: 1-chloro-3-fluoro-2-methoxybenzenewith n-butyllithium in 1,2-dimethoxyethane at -78 - -70.1; for 1.75 h;
Stage #2: with Trimethyl borate in 1,2-dimethoxyethane at -67 - 20;Further stages;Time;Temperature;
Steps:
2 Alternate Isolation of PBA from MeCN
Example 2Alternate Isolation of PBA from MeCN2,6-CFA (10.0 g, 62.28 mmol) was weighed in a separate flask and transferred to a 3-neck, 500-ml round bottom flask equipped with a thermocouple temperature probe, stir bar, and a N2 inlet. The flask was rinsed with anhydrous DME. Additional DME (total volume of 106 ml) was added to the reaction. The reaction was cooled to - 78 °C with a dry ice/acetone bath. Once the reaction reached approximately -77 °C, n- BuLi (29 ml, 71.62 mmol, 2.5 M in hexanes) was slowly added dropwise using a syringe pump over a 45 minute period. The highest temperature reached during addition was -70.1 °C. After complete addition of ?-BuLi, the reaction was left to stir for 1 hour at -72.1 °C. After 1 hour, B(OMe)3 (10.5 ml, 93.42 mmol) was added dropwise using a syringe pump over a period of 22 minutes. The highest temperature reached during the addition was -67.0 °C. After complete addition of B(OMe)3, the diy ice/ acetone bath was removed and the reaction mixture was warmed to room temperature overnight. The next morning, the reaction mixture temperature was at22.7 °C. Using an addition funnel, IN NaOH (aq) (78 ml, 77.85 mmol) was added dropwise to the reaction mixture. After complete addition, the reaction mixture was stirred for 1.5 hours at room temperature. The reaction mixture was then transferred to a 500-ml separatory funnel and the organic and aqueous layers separated. The aqueous layer was washed with TBME (2 x 75 ml) to remove unwanted impurities and/or unreacted 2,6-CFA. The aqueous layer was acidified with 6N aqueous HC1 (42 ml, 249.1 mmol) and then MeCN (3 x 75 ml) added. Since water and MeCN are miscible, the two distinct layers were not distinguishable. Solid NaCl was then added to saturate the aqueous layer, which resulted in the formation of two distinct layers: the MeCN layer and the aqueous layer, which were separated. The organic layers were combined, dried with MgS04, and filtered into a 500-ml round bottom flask. To determine the yield of the reaction, the PBA solution in MeCN was concentrated to dryness under reduced pressure. The white solid was further dried in a vacuum oven at 55 °C to give1 1.8 g (93% yield) of PBA.
References:
WO2013/16557,2013,A2 Location in patent:Page/Page column 12
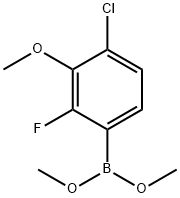
1426539-30-1
0 suppliers
inquiry

944129-07-1
81 suppliers
$45.00/25mg
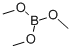
121-43-7
346 suppliers
$14.00/25mL

53145-38-3
112 suppliers
$8.00/1g

944129-07-1
81 suppliers
$45.00/25mg