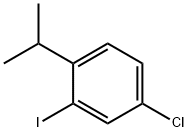
4-Chloro-2-iodo-1-isopropylbenzene synthesis
- Product Name:4-Chloro-2-iodo-1-isopropylbenzene
- CAS Number:1369923-80-7
- Molecular formula:C9H10ClI
- Molecular Weight:280.5332
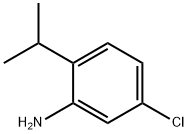
130566-32-4
3 suppliers
inquiry

1369923-80-7
11 suppliers
inquiry
Yield:1369923-80-7 77%
Reaction Conditions:
Stage #1: 5-chloro-2-isopropylanilinewith toluene-4-sulfonic acid;sodium nitrite in water; for 0.166667 h;
Stage #2: with potassium iodide in water; for 0.333333 h;
Steps:
B.4
Step 4: 4-Chloro-2-iodo-1-(propan-2-yl)benzeneA mixture of 5-chloro-2-(propan-2-yl)aniline (1.4 g, 8.3 mmol), p-toluensulfonic acid (4.7 g, 24.8 mmol) and water (0.83 mmol) were ground in a mortar for few minutes, to obtain an homogeneous paste to which solid sodium nitrite (1.42 g, 20.6 mmol) was added and the paste ground for 10 min. Solid potassium iodide (3.42 g, 20.6 mmol) was added and the paste ground for 20 min. The paste was then dissolved in water (20 mL) and treated with sodium sulfite (10% aq. sol.), before being extracted with EtOAc (3 x 50 mL). The combined organic layers were dried over _26 sodium sulfate and the crude was purified by flash chromatography (hexane) to obtain the title compound as a light- yellow oil (1.79 g, 77%).1H NMR (400 MHz, DMSO- /6) δ ppm 1.17 (d, J=6.84 Hz, 6 H), 3.08 (spt, J=6.88 Hz, 1 H), 7.33 (d, J=8A2 Hz, 1 H), 7.45 (ddd, J=8.42, 2.20, 0.37 Hz, 1 H), 7.87 (d, J=2.20 Hz, 1 H).
References:
WO2012/143248,2012,A1 Location in patent:Page/Page column 25-26

99-88-7
219 suppliers
$8.00/5g

1369923-80-7
11 suppliers
inquiry
92765-42-9
10 suppliers
inquiry

1369923-80-7
11 suppliers
inquiry

76611-16-0
7 suppliers
inquiry

1369923-80-7
11 suppliers
inquiry