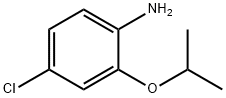
4-Chloro-2-isopropoxyaniline synthesis
- Product Name:4-Chloro-2-isopropoxyaniline
- CAS Number:99112-07-9
- Molecular formula:C9H12ClNO
- Molecular Weight:185.65
40422-90-0
6 suppliers
inquiry

99112-07-9
16 suppliers
inquiry
Yield:99112-07-9 2.25 g
Reaction Conditions:
with iron;acetic acid at 50; for 1 h;
Steps:
8 Method 32 4-Chloro-2-(propan-2-yloxy)aniline
Method 32
4-Chloro-2-(propan-2-yloxy)aniline
A mixture of 1.0 g of 4-chloro-2-fluoronitrobenzene and 9.3 g of caesium carbonate in 10 ml of 2-propanol is heated at 60° C. for 24 h.
The mixture is then concentrated and the residue is taken up with 100 ml of a mixture of water and ethyl acetate.
The aqueous phase is extracted three times with 50 ml of ethyl acetate.
The combined organic phases are washed with 50 ml of a saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered and then concentrated to dryness under reduced pressure.
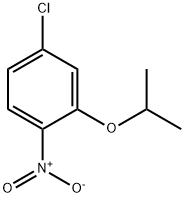
The residue is purified on 90 g of silica, elution being carried out with 95/5 heptane/ethyl acetate, so as to obtain 885 mg of 4-chloro-1-nitro-2-(propan-2-yloxy)benzene in the form of a yellow solid.
A mixture of 2.55 g of 4-chloro-1-nitro-2-(propan-2-yloxy)benzene in 40 ml of acetic acid is heated at 50° C., and then 7 ml of water and 2.64 g of iron powder are added.
The mixture is stirred for 1 h at 50° C., and then cooled to ambient temperature and filtered on Celite. The Celite is rinsed three times with 20 ml of methanol, and the filtrate is concentrated under reduced pressure.
The residue is taken up in 50 ml of 1N sodium hydroxide and 50 ml of dichloromethane.
The aqueous phase is extracted twice with 50 ml of dichloromethane.
The combined organic phases are dried over anhydrous magnesium sulfate, filtered and then concentrated to dryness under reduced pressure, so as to obtain 2.25 g of 4-chloro-2-(propan-2-yloxy)aniline in the form of a green oil.
References:
US2013/261106,2013,A1 Location in patent:Paragraph 0605