
4-chloro-2-Methylbenzofuro<3,2-d>pyriMidine synthesis
- Product Name:4-chloro-2-Methylbenzofuro<3,2-d>pyriMidine
- CAS Number:39786-40-8
- Molecular formula:C11H7ClN2O
- Molecular Weight:218.64
![2-Methyl-3H-benzo[4,5]furo[3,2-d]pyriMidin-4-one](/CAS/20150408/GIF/33149-48-3.gif)
33149-48-3
5 suppliers
inquiry

39786-40-8
39 suppliers
$10.00/100mg
Yield:39786-40-8 95%
Reaction Conditions:
with trichlorophosphate for 2 h;Reflux;
Steps:
3 Step 3 . 4-Chloro-2-methyl-benzofuro j3,2-djpyrimidine
A suspension of 2-methyl-3H-benzofuro[3,2-djpyrimidin-4-one (390.0 mg, 1.95 mmol) in phosphorus oxychloride (5.0 mL, 53.64 mmol) was heated at refluxing conditions for 2 h. to give a thick cream79orange precipitate. Anhydrous DMF (0.5 mL) was added to the give an orange solution within 5 mm. The reaction mixture was cooled to room temperature and evaporated in vacuo, to remove excess POC13 and the red liquid was poured onto crushed ice-water to give a dirty orange precipitate. The mixture was neutralized with a saturated NaHCO3 solution and the precipitate was extracted with ethyl acetate(2x 50 mL), the aqueous and the organic layers were separated, the organic layer was washed with brine (lx 25 mL) and dried over anhydrous Na2SO4, filtered and evaporated in vacuo to afford a dirty yellow-orange solid. The crude product was purified by silica-gel flash chromatography using EtOAc in hexanes to afford a colorless crystalline solid (402.5 mg, yield 95%). LC-MS analysis of the solid showed the desired product with a purity >99% and the desired product’s mass: m/z 219 (35cM+H), andm/z 221 (370M+H); Calcd for C11H7 C1N2O: 218.64. 1H NMR (400 MHz, DMSO-d6): ? 2.77 (s, 3H, CH3-), 7.54-7.63 (m, 1 H), 7.81-7.89 (m, 1H), 7.92-7.99 (m, 1H), 8.24 (dd,J= 7.82 and 0.73 Hz, 1H).
References:
WO2019/18359,2019,A1 Location in patent:Page/Page column 75; 79; 80
![2-Methyl-3H-benzo[4,5]furo[3,2-d]pyriMidin-4-one](/CAS/20150408/GIF/33149-48-3.gif)
33149-48-3
5 suppliers
inquiry

39786-40-8
39 suppliers
$10.00/100mg
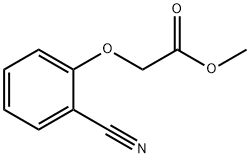
34844-79-6
13 suppliers
inquiry

39786-40-8
39 suppliers
$10.00/100mg
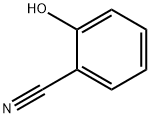
611-20-1
565 suppliers
$6.00/5g

39786-40-8
39 suppliers
$10.00/100mg
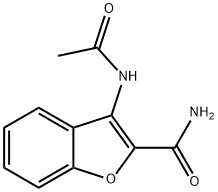
68217-77-6
2 suppliers
inquiry

39786-40-8
39 suppliers
$10.00/100mg