
4-Chloro-3-ethoxyaniline synthesis
- Product Name:4-Chloro-3-ethoxyaniline
- CAS Number:852854-42-3
- Molecular formula:C8H10ClNO
- Molecular Weight:171.62
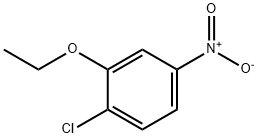
102236-22-6
14 suppliers
inquiry

852854-42-3
25 suppliers
$75.00/100mg
Yield:852854-42-3 72%
Reaction Conditions:
with iron;acetic acid in water; for 1 h;Heating / reflux;
Steps:
43
To a suspension of Description 42(6. 49 g, 32.2 mmol) in a mixture of glacial acetic acid (40 ml) and water (100 ml), mixed with an overhead stirrer was added iron powder (8. 99 g, 161 mmol) and the mixture heated at reflux for 1 hour. The mixture was cooled and filtered through hyflosupercel. The solid was washed with EtOAc (200 ml) and water (200 ml) and the organic layer separated, washed with more water (300 ml), sat. KzCOs (100ml), sat. NaCl (100 ml), dried overNa2SO4, filtered and evaporated. The residue was purified by column chromatography on silica(eluent : 100 % dichloromethane) to give a dark oil (4.01
References:
WO2005/49613,2005,A1 Location in patent:Page/Page column 53-54
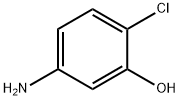
6358-06-1
175 suppliers
$5.00/250mg

75-03-6
361 suppliers
$11.00/5g

852854-42-3
25 suppliers
$75.00/100mg