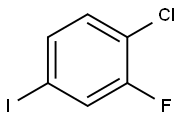
4-Chloro-3-fluorobenzaldehyde synthesis
- Product Name:4-Chloro-3-fluorobenzaldehyde
- CAS Number:5527-95-7
- Molecular formula:C7H4ClFO
- Molecular Weight:158.56
Yield:5527-95-7 81%
Reaction Conditions:
with rhodium(III) chloride trihydrate;hydrogen;triethylamine;triphenylphosphine in N,N-dimethyl acetamide at 90; under 7500.75 Torr; for 12 h;Autoclave;
Steps:
General procedure for reductive carbonylation of aryl iodides with CO and H2
General procedure: All reactions were carried out in an 80 mL Teflon-lined stainless steel reactor equipped with a magnetic stirring bar. Typically, in a glovebox, the aryl iodides (1.0 mmol), RhI3(0.025 mmol), PPh3 (0.1 mmol), Et3N (1.2 mmol), and DMA (2 mL) were loaded into the reactor. Then, the autoclave was screwed up, charged with CO and H2 to a total pressure of 10 bar (1:1) and transferred to an oil bath preheated at 90 °C, which was controlled by a Haake-D3 temperature controller. After completion of the reaction, the reactor was cooled in iced water and the gas carefully vented. The conversion and yield of the aryl iodides and arylaldehydes were determined by GC analysis using dodecane as an internal standard. For yield determination of the other products, the reaction mixture was first analyzed by GC-MS to determine the structures of the aromatic aldehyde products. Then, CH2Cl2 (5 mL) was added to the reaction mixture, after which deionized water (10 mL) was added to extract the solvent DMA for 5 times. The organic layer was dried over anhydrous Na2SO4, concentrated by rotary evaporation and finally purified by column chromatography on silica gel using n-hexane/ethyl acetate as eluent to obtain the pure products and isolated yields.
References:
Chen, Suqing;Liu, Zhenghui;Mu, Tiancheng;Wang, Peng;Yan, Zhenzhong;Yu, Dongkun;Zhao, Xinhui [Beilstein Journal of Organic Chemistry,2020,vol. 16,p. 645 - 656]
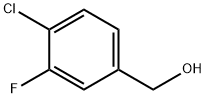
202925-10-8
73 suppliers
$6.00/1g

5527-95-7
257 suppliers
$9.00/1g
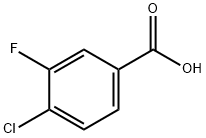
403-17-8
221 suppliers
$6.00/1g

5527-95-7
257 suppliers
$9.00/1g