
4-chloro-3-(hydroxymethyl)phenol synthesis
- Product Name:4-chloro-3-(hydroxymethyl)phenol
- CAS Number:876299-47-7
- Molecular formula:C7H7ClO2
- Molecular Weight:158.58
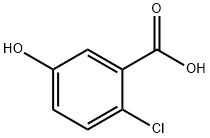
56961-30-9
139 suppliers
$18.00/1g

876299-47-7
32 suppliers
inquiry
Yield:876299-47-7 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether at 0; for 6 h;Heating / reflux;
Steps:
26 4-Chloro-3-hydroxymethyl-phenol
Lithium aluminium hydride (1M in diethyl ether, 25 mL, 25 mmol) was added to an ice-cooled solution of 2-chloro-5-hydroxy-benzoic acid (4 g, 23.2 mmol) in tetrahydrofuran (200 mL) and the mixture was heated under reflux for 6 hours. The mixture was then diluted with a mixture of water/tetrahydrofuran, acidified with 1M hydrochloric acid, and extracted with ethyl acetate. The organic solution was dried over sodium sulphate and concentrated in vacuo to afford the title compound in quantitative yield, 4.3 g.
References:
US2006/35922,2006,A1 Location in patent:Page/Page column 31