
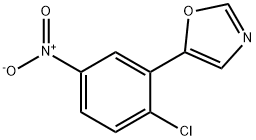
4-CHLORO-3-(OXAZOL-5-YL)ANILINE synthesis
- Product Name:4-CHLORO-3-(OXAZOL-5-YL)ANILINE
- CAS Number:521983-07-3
- Molecular formula:C9H7ClN2O
- Molecular Weight:194.62
521983-14-2
0 suppliers
inquiry

521983-07-3
14 suppliers
$310.00/100mg
Yield:521983-07-3 500 mg
Reaction Conditions:
with iron;ammonium chloride in methanol;water at 80; for 2 h;
Steps:
28
A suspension of 5-(2-chloro-5-nitrophenyl)-1,3-oxazole (800 mg, 3.6 mmol), Fe powder (806 mg, 14.4 mmol), and NH4Cl (1.56 g, 28.8 mmol) in MeOH/H2O (3:2, 15 mL) was stirred at 80 C for 2 hours, cooled to room temperature and filtered. The filtrate was concentrated, extracted with ethyl acetate, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column (EA/PE: 1/3) to give 4-chloro-3-(1,3- oxazol-5-yl)aniline (500 mg, 72%) as a white solid.1H NMR (400 MHz, DMSO-d6) δ 8.50 (s, 1H), 7.69 (s, 1H), 7.20 (d, J = 9.2 Hz, 1H), 7.03 (d, J = 2.8 Hz, 1H), 6.60 (dd, J = 2.8 Hz, 8.8 Hz, 1H), 5.50 (s, 2H). LC-MS m/z: 195.1 [M+H+]. LC-MS Purity (214 nm): > 95%; tR = 1.58 min.
References:
WO2016/73889,2016,A1 Location in patent:Paragraph 00244