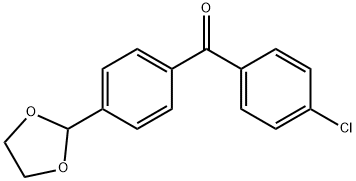
4-CHLORO-4'-(1,3-DIOXOLAN-2-YL)BENZOPHENONE synthesis
- Product Name:4-CHLORO-4'-(1,3-DIOXOLAN-2-YL)BENZOPHENONE
- CAS Number:780776-35-4
- Molecular formula:C16H13ClO3
- Molecular Weight:288.73
![Benzenemethanol, 4-chloro-α-[4-(1,3-dioxolan-2-yl)phenyl]-](/CAS/20210305/GIF/780776-32-1.gif)
780776-32-1
1 suppliers
inquiry

780776-35-4
10 suppliers
$417.00/1g
Yield:780776-35-4 99%
Reaction Conditions:
Stage #1: (4-Chloro-phenyl)-(4-[1,3]dioxolan-2-yl-phenyl)-methanolwith sodium hydrogencarbonate;sodium bromide in water;ethyl acetate at 0; for 0.5 h;
Stage #2: with sodium chlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical in water;ethyl acetate at 0; for 2 h;
Steps:
4.B B. (4-Chloro-phenyl)-(4-[1,3]dioxolan-2-yl-phenyl)-methanone.
(4-Chloro-phenyl)-(4-[1,3]dioxolan-2-yl-phenyl)-methanol (250 mg, 0.85mmol) was dissolved in ethyl acetate (1.5 mL) and treated with a solution of sodium bromide (88 mg, 0.85 mmol) in saturated aqueous NaHCO3 (2.3 mL). The reaction mixture was cooled to 0° C. and stirred for 30 min. Free radical 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) (1.3 mg, 0.008 mmol) and then aqueous sodium hypochlorite solution (1.25 mL, 0.67 M) were added, and the resulting mixture was stirred for 2 h. Ethyl acetate (100 mL) and brine (100 mL) were added, and the mixture was stirred 5 min. The organic layer was washed with brine (2*75 mL) and then dried (Na2SO4). Solvent was removed under reduced pressure yielding the title compound as an off-white solid (247 mg, 99%). TLC (silica, 50% ethyl acetate/hexanes): Rf=0.5. HPLC (Method A): Rt=9.99. MS (ESI+): mass calculated for C16H13ClO3, 288.0; m/z found, 289.0 [M+H]+. 1H NMR (500 MHz, CDCl3): δ 7.78 (d, J=8.3 Hz, 2H), 7.75 (d, J=8.6 Hz, 2H), 7.61(d, J=8.1 Hz, 2H), 7.46 (d, J=8.6 Hz, 1H), 5.89 (s, 1H), 4.16-4.12 (m, 2H), 4.11-4.06 (m, 2H).
References:
US2004/214857,2004,A1 Location in patent:Page 20