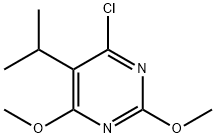
4-chloro-5-isopropyl-2,6-dimethoxypyrimidine synthesis
- Product Name:4-chloro-5-isopropyl-2,6-dimethoxypyrimidine
- CAS Number:96606-04-1
- Molecular formula:C9H13ClN2O2
- Molecular Weight:216.66

67-56-1
790 suppliers
$9.00/25ml

124-41-4
730 suppliers
$12.00/25g

1780-42-3
36 suppliers
inquiry

96606-04-1
8 suppliers
inquiry
Yield:96606-04-1 66%
Reaction Conditions:
at 0 - 20; for 18 h;Product distribution / selectivity;
Steps:
1.4; 2
Step 4 To a stirred, anhydrous methanol (600 mL) at room temperature, was added sodium metal pieces (24.7 g, 1.074 M) during 1 hr. After completion of the reaction, the solution was cooled in an ice bath and 5-isopropyl-2,4,6-trichloropyrimidine (121.08 g, 0.537 M) was added portion-wise (over 20-25 min.). After 1 hr., the ice bath was removed and the mixture was stirred at room temperature overnight. The mixture was evaporated in vacuo and the residue was dissolved in ether, washed with water, dried with MgSO4, filtered, and evaporated in vacuo. The residue was purified by silica gel column chromatography (eluent: 3:97 ether/hexane) to afford 105 g (90%) of 6-chloro-2,4-dimethoxy-5-isopropylpyrimidine (4) as a colorless oil. m.p. 23-24° C.; 1H NMR (200 MHz, CDCl3) δ1.23 (6H, d, J=7.1 Hz), 3.39 (1H, m), 3.94 (3H, s), 3.97 (3H, s); 13C NMR (50 MHz, CDCl3) δ19.61, 27.30, 54.19, 54.83, 117.33, 158.59, 161.69, 170.05; m/z(EI) 216(M+) 6-Chloro-2,4-dimethoxy-5-isopropylpyrimidine; To a stirred solution of 2,4,6-trichloropyrimidine (4.24 kg, 18.80 mol) in methanol (40 L), sodium methoxide was added portion-wise (2.11 kg 39.06 mol) at 0-10° C. The reaction mixture was stirred at room temperature for 18 hours. The mixture was evaporated in vacuo and the residue was dissolved in n-hexane (20 L). The hexane solution was washed with water (10 L), dried with MgSO4 (1 kg) and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane, 1:15) to give a pale brown oil. (2.72 kg, 12.55 mol, 66% yield)1H NMR (300 MHz, CDCl3) δ 1.23 (6H, d, J=7.1 Hz), 3.39 (1H, m), 3.94 (3H, s), 3.97 (3H, s); m/z (EI) 216(M+)
References:
US2009/163712,2009,A1 Location in patent:Page/Page column 13-14; 18