
4-Chloro-5-isopropyl-5H-pyrrolo[3,2-d]pyrimidine synthesis
- Product Name:4-Chloro-5-isopropyl-5H-pyrrolo[3,2-d]pyrimidine
- CAS Number:919278-25-4
- Molecular formula:C9H10ClN3
- Molecular Weight:195.65
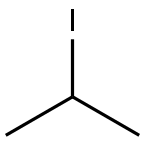
75-30-9
259 suppliers
$15.00/1g
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
200 suppliers
$9.00/250mg
![4-Chloro-5-isopropyl-5H-pyrrolo[3,2-d]pyrimidine](/CAS/20180703/GIF/919278-25-4.gif)
919278-25-4
10 suppliers
inquiry
Yield:919278-25-4 75%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20; for 72 h;
Steps:
25
A mixture of 4-chloro-5H-pyrrolo [3, 2-d] pyrimidine (2.30 g, 15 mmol), 2-iodopropane (2.81 g, 16.5 mmol), cesium carbonate (9.77 g, 30 mmol) and N, N-dimethylformamide (15 mL) was stirred at room temperature for 3 days. The reaction mixture was concentrated under reduced pressure, and the residue was diluted with water and extracted with ethyl acetate. The organic layer was concentrated under reduced pressure, and the residue was purified by column chromatography (silica gel, hexane/ethyl acetate=90/10->0/100) to give the title compound (2.20 g, 75%) as a white solid. 1H-NMR (DMSO-d6, 300 MHz) δ 1-53 (6H, d, J = 6.7 Hz), 5.41 (IH, EPO
References:
WO2007/4749,2007,A1 Location in patent:Page/Page column 121-122