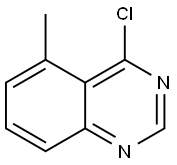
4-chloro-5-methylquinazoline synthesis
- Product Name:4-chloro-5-methylquinazoline
- CAS Number:90272-82-5
- Molecular formula:C9H7ClN2
- Molecular Weight:178.62
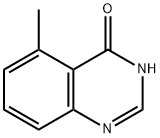
75844-41-6
50 suppliers
$45.00/50mg

90272-82-5
24 suppliers
$165.00/0.25g
Yield:90272-82-5 85%
Reaction Conditions:
with 2,2'-iminobis[ethanol];trichlorophosphate in toluene; for 0.5 h;Heating / reflux;
Steps:
1.B
1B. Preparation of 4-chloro-5-methylquinazoline To a solution of 1A (1.44 g, 9.0 mmol) in toluene (24 ml) was added POCl3 (13.5 ml) and DEA (3.6 ml). The mixture was refluxed under N2 for 30 min. After cooling to room temperature, the reaction mixture was concentrated under vacuum. The residue was dissolved in EtOAc, washed with 10% citric acid solution, followed by saturated NaHCO3. The residue was dried with MgSO4, filtered, and concentrated to give 1B (1.37 g, 85%) as a solid. 1H-NMR (400 MHz, CDCl3): 8.96 (s, 1H), 7.94 (d, J=8.52 Hz, 1H), 7.80 (dd, J=7.17 Hz, J=8.52 Hz, 1H), 7.51 (d, J=7.17 Hz, 1H), 3.05 (s, 3H).
References:
US2006/217369,2006,A1 Location in patent:Page/Page column 10
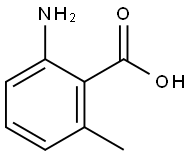
4389-50-8
270 suppliers
$6.00/1g

90272-82-5
24 suppliers
$165.00/0.25g

13506-76-8
246 suppliers
$6.00/1g

90272-82-5
24 suppliers
$165.00/0.25g