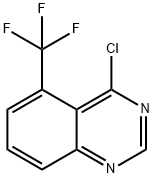
4-Chloro-5-(trifluoromethyl)quinazoline synthesis
- Product Name:4-Chloro-5-(trifluoromethyl)quinazoline
- CAS Number:16499-63-1
- Molecular formula:C9H4ClF3N2
- Molecular Weight:232.59

436-73-7
5 suppliers
inquiry

16499-63-1
31 suppliers
inquiry
Yield:16499-63-1 91.38%
Reaction Conditions:
with triethylamine;trichlorophosphate at 0; for 2 h;Reflux;
Steps:
2 Step 2 : synthesis of 4-chloro-5 -(trifluoromethyl)quinazo line 14
Triethyl amine (29.3 ml, 210 mmol) was added to a mixture of intermediate 14-b (8.00 g,37.4 mmol) in phosphorus oxychloride (331 g, 2.16 mol) at 0°C. The mixture was refluxed for 2 hours. The solvent was evaporated under vacuum. The residue was dissolved in ethyl acetate (200 ml) and the mixture was added to ice (200 g). The separated organic layer was washed successively with water (1 x 100 ml), 10% sodium bicarbonate aqueous solution (2x 100 ml), water (1 xlOO ml) and brine (1 x 100 ml). The separated organic layer was dried over sodium sulfate, filtered and concentrated under vacuum. The residue was purified by column chromatography over silica gel (eluent: petroleum ether/ethyl acetate 1/0 to 1 / 1) to give intermediate 14 (7.97 g, 91.38%).1H NMR (400 MHz, CDC13) ? ppm 7.89 - 8.06 (m, 1 H) 8.22 (d, J=7.50 Hz, 1 H) 8.31 (d,J=8.38 Hz, 1 H) 9.11 (s, 1 H)
References:
WO2016/91791,2016,A1 Location in patent:Page/Page column 26; 27
314-46-5
80 suppliers
$40.00/100mg

16499-63-1
31 suppliers
inquiry