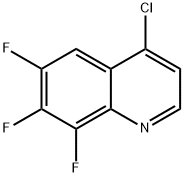
4-Chloro-6,7,8-trifluoroquinoline synthesis
- Product Name:4-Chloro-6,7,8-trifluoroquinoline
- CAS Number:1020087-33-5
- Molecular formula:C9H3ClF3N
- Molecular Weight:217.57
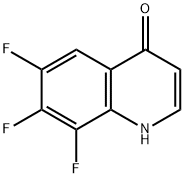
1020087-32-4
2 suppliers
inquiry

1020087-33-5
5 suppliers
inquiry
Yield:1020087-33-5 54%
Reaction Conditions:
Stage #1: 6,7,8-trifluoroquinolin-4(1H)-onewith trichlorophosphate for 1.3 h;Heating / reflux;
Stage #2: with ammonia in dichloromethane;water;
Steps:
KK.iii
4-Chloro-6,7,8-trifluoroquinoline (G4). A solution of quinolone G3 (1.30 g, 6.53 mmol) was refluxed in POCI3 (50 mL) for 1.3 h, and then excess POCI3 was removed under reduced pressure. The residue was re- dissolved in CH2CI2, and the resulting solution basified by addition of aqueous ammonia solution. The resulting solution was extracted with CH2CI2, and the combined organic extracts washed sequentially with H2O and brine, and dried over MgSO4. Solvent was removed under reduced pressure, and the residue purified by flash chromatography on silica gel, eluting with 100% CH2CI2→1% MeOH:CH2CI2→10% MeOH:CH2CI2), to give trifluoroquinoline G4 (0.76 g, 54%) as a white crystalline solid; mp 119-121 0C; 1H NMR (400 MHz, DMSO): δ 8.93 (d, J = 4.73 Hz, 1 H, ArH), 8.08 (ddd, JH-F = 10.23, 7.92, 2.30 Hz, 1 H, ArH), 7.95 (d, J = 4.73 Hz, 1 H, ArH); LCMS (APCI+): 218 (100%), 220 (100%).
References:
WO2008/46085,2008,A2 Location in patent:Page/Page column 114

79660-46-1
98 suppliers
$18.00/10g

1020087-33-5
5 suppliers
inquiry
151391-68-3
0 suppliers
inquiry

1020087-33-5
5 suppliers
inquiry