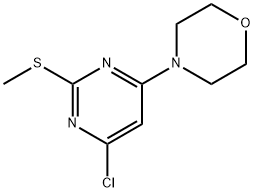
4-CHLORO-6-MORPHOLINO-2-PYRIMIDINYL METHYL SULFIDE synthesis
- Product Name:4-CHLORO-6-MORPHOLINO-2-PYRIMIDINYL METHYL SULFIDE
- CAS Number:339016-21-6
- Molecular formula:C9H12ClN3OS
- Molecular Weight:245.73
Yield: 98%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 60; for 18 h;
Steps:
21
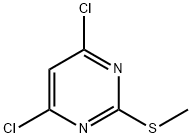
Reference Example 21 6-Fluoro-4-(2-methanesulfonyl-6-morpholin-4-yl-pyrimidin- 4-vIMH-indole; To a stirred solution of 4,6-dichloro-2-(methylthio)pyrimidine (15.44 g; 79 mmol) and DIPEA (15 ml; 86 mmol) in THF (200 ml) at RT. was added morpholine (7.6 mL; 87 mmol) in one portion (a thick white precipitate forms within a few minutes). The reaction mixture
References:
PIRAMED LIMITED;THE INSTITUTE OF CANCER RESEARCH WO2008/125839, 2008, A2 Location in patent:Page/Page column 24; 37-38