
4-chloro-7-ethoxyquinoline synthesis
- Product Name:4-chloro-7-ethoxyquinoline
- CAS Number:178984-50-4
- Molecular formula:C11H10ClNO
- Molecular Weight:207.66
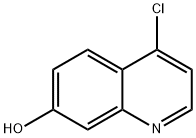
181950-57-2
87 suppliers
$80.00/0.25g

74-96-4
427 suppliers
$9.00/5g

178984-50-4
19 suppliers
inquiry
Yield:178984-50-4 67.2%
Reaction Conditions:
Stage #1: 4-chloro-7-hydroxyquinolinewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 20; for 0.25 h;
Stage #2: ethyl bromide in N,N-dimethyl-formamide;mineral oil;
Steps:
49 Example 49: Preparation of 4-chloro-7-ethoxyquinoline (Compound 23a)
To a 100 ml round bottom flask was added 4-chloro-7-hydroxyquinoline (0.72 g, 4 mmol)DMF (10 ml) and60%NaH (0.4 g, 10 mmo 1),Stir at room temperature for 15 min, add 1-bromoethane (20 mmo 1) to continue the reaction, TLC follow-up test.After completion of the reaction, the reaction mixture was poured into water and extracted with ethyl acetate. The organic phases were combined, washed with water, washed with saturated brine,Dried over sodium, dried under reduced pressure to dryness and chromatographed on silica gel (mobile phase: dichloromethane / methanol = 200/1) to give a pale yellow solid (0.56 g, yield 67.2%)
References:
CN105037266,2017,B Location in patent:Paragraph 0323-0324; 0327-0328

181950-57-2
87 suppliers
$80.00/0.25g

64-17-5
706 suppliers
$10.00/50g

178984-50-4
19 suppliers
inquiry
68500-37-8
198 suppliers
$18.00/250mg

178984-50-4
19 suppliers
inquiry

82121-05-9
169 suppliers
$58.00/5g

178984-50-4
19 suppliers
inquiry

536-90-3
289 suppliers
$10.00/5g

178984-50-4
19 suppliers
inquiry