
4'-CHLORO-BIPHENYL-3-CARBOXYLIC ACID synthesis
- Product Name:4'-CHLORO-BIPHENYL-3-CARBOXYLIC ACID
- CAS Number:4655-10-1
- Molecular formula:C13H9ClO2
- Molecular Weight:232.66
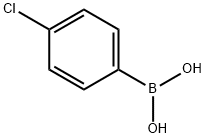
1679-18-1
585 suppliers
$10.00/1g
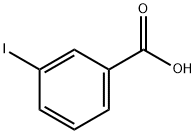
618-51-9
386 suppliers
$9.00/10g

4655-10-1
67 suppliers
$55.00/250mg
Yield: 99%
Reaction Conditions:
with potassium carbonate in ethanol;water at 180; for 0.25 h;Microwave heating;
Steps:
1
The carboxylic acids 2, 4, 6, and 8 were created by the following general procedure: 3- or 4-iodobenzoic acid (1.24 g, 5 mmol), K2CO3 (2.7 g, 20 mmol) and the appropriate boronic acid (4-chlorophenyl (0.89 g, 5.5 mmol) or 2-naphthyl (0.95 g, 5.5 mmol)) were dissolved in a mixture Of H2O (7.5 ml) and ethanol (7.5 Ml) and heated to 180 °C for 15 min using microwave heating. The reaction mixture was filtered through a plug of Celite, HCl (IM, 25 mL) was added to the filtrate and the resulting mixture was extracted twice with EtOAc. The organic phases were combined, washed with water (50 mL) and brine (50 mL) and concentrated to yield a crude solid, which was recrystallized from toluene to afford the desired compound as a white solid. Scheme 3 below illustrates the creation of the carboxylic acids.; [0164] 3-Iodobenzoic acid and 4-chlorophenylboronic acid yielded 1.2 g (99%) of 2. Mp 180.9 - 181.7 0C. IH NMR (DMSO-d6) δ 7.51 (d, 2H, J = 7.7 Hz), 7.58 (dd, IH, J = 7.7, 8.2 Hz), 7.71 (d, 2H, J = 7.7 Hz), 7.88 (d, IH, J = 8.2 Hz), 7.96 (d, IH, J = 7.7 Hz), 8.19 (s, IH). 13C NMR (DMSO-d6) δ 127.8, 128.8, 129.0 (2 C:s), 129.5 (2 C:s), 129.9, 131.4, 132.5, 133.3, 138.6, 139.7, 168.2.
References:
ACADIA PHARMACEUTICALS INC. WO2008/57543, 2008, A2 Location in patent:Page/Page column 42-43