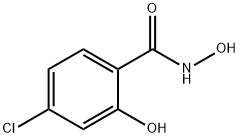
4-CHLORO-N,2-DIHYDROXYBENZAMIDE synthesis
- Product Name:4-CHLORO-N,2-DIHYDROXYBENZAMIDE
- CAS Number:61799-78-8
- Molecular formula:C7H6ClNO3
- Molecular Weight:187.58
22717-55-1
74 suppliers
$12.00/1g

61799-78-8
30 suppliers
$53.02/250mgs:
Yield:61799-78-8 88%
Reaction Conditions:
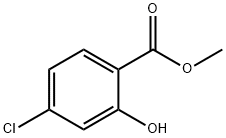
Stage #1: methyl 4-chloro-2-hydroxybenzoatewith hydroxylamine hydrochloride;sodium hydroxide in ethanol;water at 20; for 24 h;Cooling with ice;Inert atmosphere;
Stage #2: with hydrogenchloride for 0.5 h;Reflux;
Steps:
1.2
2) Preparation of 4-chloro-N,2-dihydroxy-benzamide Hydroxylamine hydrochloride in solid (5.25g, 75 mmol) is placed in an egg type flask and dissolved by drop-adding small amount of water in ice-bath. Thereafter, thereto is drop-added 15 ml of 50% NaOH solution. The reaction is stirred for 5 minutes and is drop-added 50 ml of dioxane solution of methyl 4-chloro-salicylate (9.3g, 50 mmol) under coverage of N2. After completion of drop-addition, a reaction is carried out at room temperature for 24 hours, and then a red-brown deposit precipitates, which is filtered out and placed in an egg type flask, thereto is added 50 ml of 10% hydrochloric acid and then reflux for 0.5 hour. After cooling to room temperature, a yellow deposit precipitates, which is filtered out and recrystallized in anhydrous ethanol, to obtain 8.25g of 4-chloro-N,2-dihydroxy-benzamide, with a yield of 88%.
References:
EP2322520,2011,A1 Location in patent:Page/Page column 12
1800-54-0
0 suppliers
inquiry

22717-55-1
74 suppliers
$12.00/1g

61799-78-8
30 suppliers
$53.02/250mgs:

1643-78-3
13 suppliers
inquiry

5106-98-9
264 suppliers
$6.00/5g

61799-78-8
30 suppliers
$53.02/250mgs: