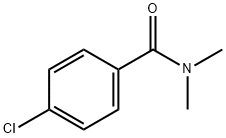
4-chloro-N,N-dimethylbenzamide synthesis
- Product Name:4-chloro-N,N-dimethylbenzamide
- CAS Number:14062-80-7
- Molecular formula:C9H10ClNO
- Molecular Weight:183.63

124-40-3
498 suppliers
$18.00/100ml
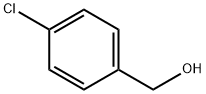
873-76-7
339 suppliers
$6.00/10g

14062-80-7
46 suppliers
$15.00/5mg
Yield: 88%
Reaction Conditions:
with tert.-butylhydroperoxide;[bis(acetoxy)iodo]benzene in acetonitrileReflux;
Steps:
General procedure for the oxidative amidation of aryl alcohol with secondary amine
General procedure: To a mixture of aryl alcohol (1.0 mmol), appropriate amine (2.5 mmol) and TBHP (70 wt % in H2O, 8.0 mmol) in acetonitrile (5 mL), was added DIB (0.2 mmol, 20 mol %) at room temperature. The reaction was allowed to stir at reflux temperature for 5-6 h. After the reaction was complete, as indicated by TLC, the mixture was cooled to room temperature. The volatiles were removed under reduced pressure and 10 mL saturated solution of NaHCO3 was added. The mixture was extracted with ethylacetate (310 mL). The combined organic phases were dried with Na2SO4 and evaporated under vacuum. Purification of the residue by column chromatography (petroleum ether/EtOAc) afforded the desired amide.
References:
Sutar, Yogesh B.;Bhagat, Saket B.;Telvekar, Vikas N. [Tetrahedron Letters,2015,vol. 56,# 48,p. 6768 - 6771]
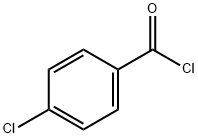
122-01-0
368 suppliers
$13.00/25g

124-40-3
498 suppliers
$18.00/100ml

14062-80-7
46 suppliers
$15.00/5mg
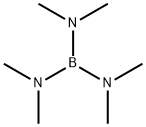
4375-83-1
44 suppliers
$95.00/1g
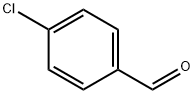
104-88-1
612 suppliers
$5.00/25g

14062-80-7
46 suppliers
$15.00/5mg
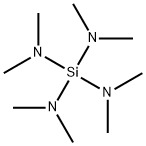
1624-01-7
69 suppliers
$23.80/1g

104-88-1
612 suppliers
$5.00/25g

14062-80-7
46 suppliers
$15.00/5mg
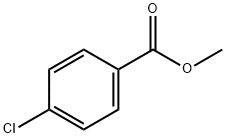
1126-46-1
227 suppliers
$8.00/25g
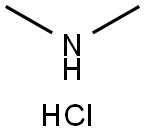
506-59-2
528 suppliers
$5.00/5 g

14062-80-7
46 suppliers
$15.00/5mg