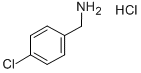
4-Chlorobenzylamine hydrochloride synthesis
- Product Name:4-Chlorobenzylamine hydrochloride
- CAS Number:42365-43-5
- Molecular formula:
- Molecular Weight:0

623-03-0
440 suppliers
$6.00/25g

42365-43-5
24 suppliers
$904.00/5g
Yield:42365-43-5 99%
Reaction Conditions:
Stage #1: 4-Cyanochlorobenzenewith [2,6-η6:η1-bis(2,4,6-trimethylphenyl)phenylthiolato]triethylphosphineruthenium(II)tetrakis[3,5-bis(trifluoromethyl)phenyl]borate;diethylphenylsilane at 20; for 18 h;Glovebox;Inert atmosphere;
Stage #2: with hydrogenchloride in diethyl ether at 20; for 1 h;Glovebox;Inert atmosphere;
Steps:
2.1.2 General Procedure for Nitrile-to-Amine Reduction Catalyzed by [3a]+[BArF4]- (GP2)
General procedure: In a glove box, a flame-dried GLC vial equipped with a magnetic stir bar is charged with[3a]+[BArF4]- (1.0 mol%) and Me2PhSiH (2a) (2.1 or 5.0 equiv). The indicated nitrile is added eitherin the glove box (for solid starting materials) or by micro syringe outside the glove box, and theresulting reaction mixture is maintained at room temperature for the indicated time. The reaction isquenched by the addition of a mixture of cyclohexane and tert-butyl methyl ether (90:10) containing4% Et3N (0.5 mL), and the resulting solution is filtered through a pad of Celite coated by a smalllayer of silica gel with a solution of cyclohexane and tert-butyl methyl ether (90:10) containing 4%Et3N (3-4 mL) as eluent. Solvents are removed under reduced pressure, and the residue isdissolved in Et2O (1 mL) followed by addition of HCl (2M in Et2O, 1.0 mL, 2.0 mmol, 10 equiv). Theresulting suspension is stirred for 1 h and filtered, affording the amines as hydrochloride salts aswhite to yellow solids.
References:
Wübbolt, Simon;Oestreich, Martin [Synlett,2017,vol. 28,# 18,p. 2411 - 2414] Location in patent:supporting information
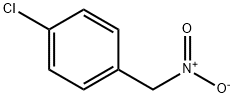
29559-24-8
6 suppliers
inquiry

42365-43-5
24 suppliers
$904.00/5g

104-86-9
277 suppliers
$6.00/5g

42365-43-5
24 suppliers
$904.00/5g

619-56-7
196 suppliers
$30.00/25g

42365-43-5
24 suppliers
$904.00/5g

27032-10-6
12 suppliers
$45.00/50mg

42365-43-5
24 suppliers
$904.00/5g