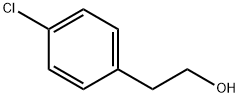
4-Chlorophenethylalcohol synthesis
- Product Name:4-Chlorophenethylalcohol
- CAS Number:1875-88-3
- Molecular formula:C8H9ClO
- Molecular Weight:156.61

1073-67-2
298 suppliers
$7.00/5g

1875-88-3
267 suppliers
$6.00/5g
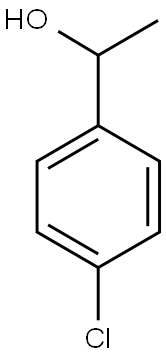
3391-10-4
137 suppliers
$40.60/10 g
Yield:1875-88-3 76 %Spectr. ,3391-10-4 13 %Spectr.
Reaction Conditions:
Stage #1:4-vinylbenzyl chloride with sodium tetrahydroborate;ethyl iodide in 1,2-dimethoxyethane at 25; for 20 h;
Stage #2: with dihydrogen peroxide;sodium hydroxide in water at 0 - 25; for 0.333333 h;Overall yield = 89 percent;Solvent;Reagent/catalyst;
Steps:
Typical procedure for hydroboration of alkenes with borane from NaBH4 and ethyl iodide using the PV method (Condition C)
General procedure: Ethyl iodide (380 mg, 2.4 mmol) and magnetic stirring bar were placed at the bottom of a test tube (15mm f × 130 mm), to which Galden HT-135 (2 mL) and Galden HT-200 (1 mL) were added slowly using a syringe in order. Subsequently, NaBH4 (84 mg, 2.2 mmol), a solution of 4-methylstyrene (1a) (240mg, 2.0 mmol) in DME (5.0 mL) were added slowly in order, forming four layers. A rubber septum was fitted to the test tube, and a needle equipped with a balloon, which acted as a reservoir of borane gas during the reaction, was then pricked into the septum. The air in the test tube was removed by a syringe until the balloon was completely flattened. The test tube was stirred slowly for 20 h at 25 °C, taking care not to mix the layers. The reaction mixture was then cooled to 0 °C with ice while stirring. An aqueous NaOH solution (1 M, 1.35 mL) was added slowly in ten portions. Verifying that organic layer was changed to basic condition using a pH test paper, H2O2 solution (30%, 0.5 mL) was added slowly in two portions. After 20 min stirring, the organic layer was taken up with a glass pipette to a separating funnel, and washed with water and brine three times. The organic layer was dried over Na2SO4. After filtration, the solvent was evaporated. The residue was then purified by column chromatography on silica gel,eluting hexane/ethyl acetate (8:2) afforded 2-(p-tolyl)-1-ethanol (2a) (247 mg, 91%).
References:
Kawamoto, Takuji;Matsubara, Hiroshi;Ryu, Ilhyong;Sato, Aoi;Soga, Nene;Yoshiki, Tomo [Tetrahedron Letters,2021,vol. 69,art. no. 152977] Location in patent:supporting information
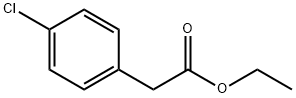
14062-24-9
114 suppliers
$6.00/10g

1875-88-3
267 suppliers
$6.00/5g
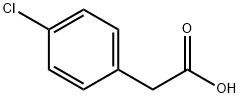
1878-66-6
446 suppliers
$6.00/25g

1875-88-3
267 suppliers
$6.00/5g
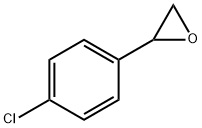
2788-86-5
67 suppliers
$40.00/100mg

1875-88-3
267 suppliers
$6.00/5g
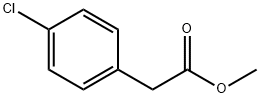
52449-43-1
185 suppliers
$6.00/5g

1875-88-3
267 suppliers
$6.00/5g