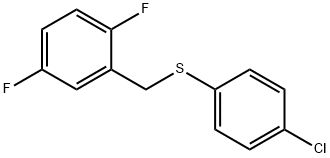
(4-chlorophenyl)(2,5-difluorobenzyl)sulfane synthesis
- Product Name:(4-chlorophenyl)(2,5-difluorobenzyl)sulfane
- CAS Number:470716-52-0
- Molecular formula:C13H9ClF2S
- Molecular Weight:270.73

106-54-7
242 suppliers
$10.00/5g
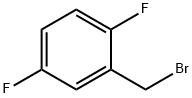
85117-99-3
238 suppliers
$7.00/1g

470716-52-0
8 suppliers
$437.00/1g
Yield:470716-52-0 99.6%
Reaction Conditions:
Stage #1: p-Chlorothiophenolwith sodium hydroxide in industrial methylated spirits;water at 20;
Stage #2: 2-(bromomethyl)-1,4-difluorobenzene in industrial methylated spirits;water at 15;
Steps:
1 Example 1; 2-[[(4-chlorophenyl)thio]methyl]-1,4-difluorobenzene
4-Chlorothiophenol (253g, 1. [75MOL)] was dissolved in industrial methylated spirits (1265mL) and 2M sodium hydroxide solution [(901ML)] was added, maintaining the temperature below [20°C.] A solution of 2,5- difluorobenzyl bromide (355g, 1. [72MOL)] in industrial methylated spirits (250mL) was added dropwise to the [THIOLATE] solution, maintaining the temperature below [15°C.] Upon completion of the reaction, water (lOOOmL) was added. The resulting slurry was aged at [5°C] and then filtered. The cake was washed sequentially with cold industrial methylated spirits: water (40: 60) and then water (500mL). Drying [IL7.] vacuo at ambient temperature furnished 2- [ [ (4-chlorophenyl) thio] methyl]- 1, [4-DIFLUOROBENZENE] (462.3g, 99. [6%). 1H] NMR [(CDC13)] 7.23 (4H, s), 6.69- 6.86 (3H, [M)] and 4.04 (2H, s).
References:
WO2004/13090,2004,A1 Location in patent:Page 11

106-54-7
242 suppliers
$10.00/5g
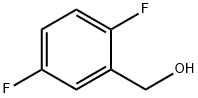
75853-20-2
149 suppliers
$13.00/5g

470716-52-0
8 suppliers
$437.00/1g