
4-Chloropicolinic Acid Hydrochloride synthesis
- Product Name:4-Chloropicolinic Acid Hydrochloride
- CAS Number:1036648-06-2
- Molecular formula:C6H5Cl2NO2
- Molecular Weight:194.01
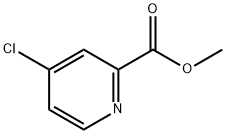
24484-93-3
401 suppliers
$6.00/5g

1036648-06-2
16 suppliers
$446.00/250mg
Yield:1036648-06-2 55%
Reaction Conditions:
Stage #1: 4-Chloro-pyridine-2-carboxylic acid methyl esterwith lithium hydroxide;water in tetrahydrofuran at 20;
Stage #2: with hydrogenchloride;water
Steps:
2.4
Example 2.4: Preparation of 3-Hydroxy-8-morpholin-4-yl-4-oxo-4//-pyrido[l,2- α]pyrimidine-2-carboxylic acid methyl ester; rt t-Butanol 2-Picolinic acid was reacted with thionyl chloride and methanol to provide methyl 4- chloro-2-picolinate which was hydrolysed and the hydrochloride salt and subjected to a Curtius rearrangement. Cleavage of the Boc protecting group afforded 2-amino-4- chloropyridine. The procedure described in W02006040520 was adapted to introduce the morpholine at position 4. This was cyclised to the intermediate ester using an adaptation of the procedure described in Example 2 where the reaction was performed at 60° C.1H NMR (300 MHz, DMSO-/) δ 3.43 (t, J=4.7 Hz, 4H), 3.73 (t, J=4.7 Hz, 4H), 3.85 (s, 3H), 6.67 (d, J=2.4 Hz, 1H),7.27 (dd, J=8.2 Hz, 2.5 Hz, IH), 8.59 (d, J=8.2 Hz, IH), 9.29-9.63 (brs, IH) MS (ESI+) m/z 328 (M+23)
References:
WO2008/77188,2008,A1 Location in patent:Page/Page column 28
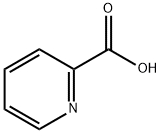
98-98-6
602 suppliers
$5.00/10g

1036648-06-2
16 suppliers
$446.00/250mg