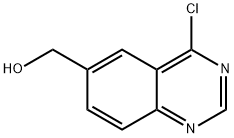
(4-chloroquinazolin-6-yl)methanol synthesis
- Product Name:(4-chloroquinazolin-6-yl)methanol
- CAS Number:648449-06-3
- Molecular formula:C9H7ClN2O
- Molecular Weight:194.62
152536-17-9
76 suppliers
$45.00/10mg

648449-06-3
18 suppliers
$265.63/250mg
Yield:648449-06-3 66%
Reaction Conditions:
with diisobutylaluminium hydride in tetrahydrofuran at -25 - 20; for 2 h;
Steps:
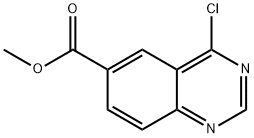
12.I Step [I] : [4-CHLOROQUINAZOLINE-6-YL] methanol
To a solution of [METHYL-4-CHLOROQUINAZOLINE-6-CARBOXYLATE] (3. 5g, [0.] [015MOL)] in dry THF (35mL) at-25°C was added DIBAL-H (4.4g, 0. [031MOL)] and stirred at-25°C to RT for 2h. The reaction mixture was cooled [TO-10°C] and quenched with 10% aqueous [NAHC03] (9mL). The reaction mixture was extracted with ethylacetate [(LOOML),] washed with water, brine and dried. The solvent was removed under vacuum to give 4-chloroquinoline-6-yl methanol (2g, 66%).
References:
WO2004/7491,2004,A1 Location in patent:Page 59-60