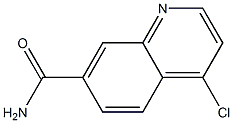
4-chloroquinoline-7-carboxaMide synthesis
- Product Name:4-chloroquinoline-7-carboxaMide
- CAS Number:178984-42-4
- Molecular formula:C10H7ClN2O
- Molecular Weight:206.6284
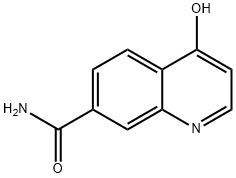
220998-68-5
7 suppliers
inquiry

178984-42-4
7 suppliers
inquiry
Yield:178984-42-4 52%
Reaction Conditions:
with trichlorophosphate at 140; for 0.5 h;
Steps:
4-Chloroquinoline-7-carboxamide (7d)
6d (1.05 g, 5.58 mmol) was treated with excess phosphorus oxychloride (4 mL) and heated at 140 °C for 30 minutes. The mixture was then left to cool to room temperature, added into ice and neutralised with 2 M NaOH. The resultant precipitate was filtered, washed with water and dried. 7d (60 mg, 52%) was obtained as white powder, mp 289 -291 °C. δH (400 MHz; CDCl3) 6.08 (1H, d, J 7.4 Hz, H-3), 7.73 (1H, dd, J 1.6, 8.4 Hz, H-6), 7.93 (1H, d, J 8.4 Hz, H-5), 8.02 (1H, d, J 1.4 Hz, H-8), 8.13 (1H, d, J 7.5 Hz, H-2). δC (100 MHz; CDCl3) 119.1 (C-3), 123.2 (C-4a), 124.7 (C-5), 127.1 (C-6), 127.9 (C-8), 130.0 (C-7), 131.2 (C-8a), 140.0 (C-4), 154.2 (C-2), 164.1 (-CONH2).
References:
Nsumiwa, Samkele;Kuter, David;Wittlin, Sergio;Chibale, Kelly;Egan, Timothy J. [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 13,p. 3738 - 3748] Location in patent:supporting information
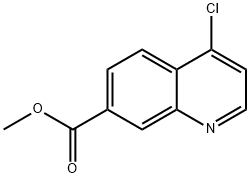
178984-69-5
45 suppliers
$65.00/100mg

178984-42-4
7 suppliers
inquiry
3544-24-9
229 suppliers
$5.00/100mg

178984-42-4
7 suppliers
inquiry