
4-Cyanophenethyl Methanesulfonate synthesis
- Product Name:4-Cyanophenethyl Methanesulfonate
- CAS Number:119744-42-2
- Molecular formula:C10H11NO3S
- Molecular Weight:225.26
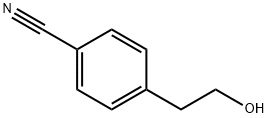
69395-13-7
108 suppliers
$12.00/250mg
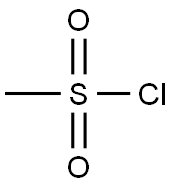
124-63-0
6 suppliers
$10.00/5g

119744-42-2
5 suppliers
inquiry
Yield:119744-42-2 100%
Reaction Conditions:
with triethylamine in dichloromethane;
Steps:
19.a a
a 1-(2-(4-Cyanophenyl)ethyl)methane sulphonate Methane sulphonyl chloride (0.856 g, 7.47 mmol) was added to a solution of 2-(4-cyanophenyl)ethanol (1.0 g, 6.80 mmol) and triethylamine (1.03 g, 10.18 mmol), in CH2 Cl2 (30 ml), at +5° C. The mixture was warmed to room temperature and stirred for 2 h before adding H2 O (15 ml), basifying with solid K2 CO3 and extracting with CH2 Cl2 (*3). The combined extracts were dried (Na2 SO4) and evaporated to afford the title-mesylate (1.52 g, 100%), δ (360 MHz, d6 -DMSO) 3.10 (2H, t, J=6.5Hz, CH2 -OMs), 3.12 (3H, s, OMs), 4.46 (2H, t, J=6.5Hz, CH2), 7.52 (2H, d, J=8.2Hz, Ar-H), 7.80 (2H, d, J=8.2Hz, Ar-H).
References:
US5807857,1998,A

69395-13-7
108 suppliers
$12.00/250mg
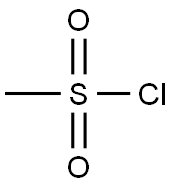
124-63-0
6 suppliers
$10.00/5g

119744-42-2
5 suppliers
inquiry