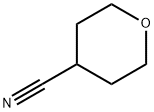
4-Cyanotetrahydro-4H-pyran synthesis
- Product Name:4-Cyanotetrahydro-4H-pyran
- CAS Number:4295-99-2
- Molecular formula:C6H9NO
- Molecular Weight:111.14
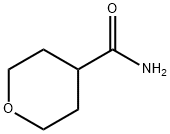
344329-76-6
116 suppliers
$7.00/250mg

4295-99-2
166 suppliers
inquiry
Yield:4295-99-2 94%
Reaction Conditions:
with thionyl chloride for 4 h;Reflux;Inert atmosphere;
Steps:
Oxane-4-carboxamide and oxane-4-carbonitrile, 20
Thionyl chloride (10.0 mL, 137 mmol) was added to oxane-4-carboxamide (3.0 g, 23 mmol) and thereaction mixture was refluxed for 4 h, after which the reaction mixture was poured over ice andbasified to pH 14 with NaOH (50%). The aqueous solution was extracted with EtOAc (3 × 50 mL),dried (Na2SO4) and concentrated in vacuo to give the product as a pale yellow oil (2.4 g, 94%). Theproduct obtained did not require any further purification.IR νmax 2961, 2932, 2851, 2240, 1468, 1446, 1390, 1242, 1125, 1066, 1011 cm-1;1H-NMR (CDCl3, 500 MHz): 3.91 (2H, ddd, J 11.9, 6.3, 3.6, 2×2-HA), 3.61 (2H, ddd, J 11.9, 7.8, 3.3,2×2-HB), 2.91-2.85 (1H, m, 4-H), 1.99-1.92 (2H, m, 2×3-HA), 1.91-1.84 (2H, m, 2×3-HB);13C-NMR (CDCl3, 75 MHz): 121.2, 65.6, 28.9, 25.3;HRMS (ESI+): Calculated for C6H10NO ([M+H]+): 112.0756. Found: 112.0754, Δ -1.8 ppm.
References:
Craven, Philip;Aimon, Anthony;Dow, Mark;Fleury-Bregeot, Nicolas;Guilleux, Rachel;Morgentin, Remy;Roche, Didier;Kalliokoski, Tuomo;Foster, Richard;Marsden, Stephen P.;Nelson, Adam [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 11,p. 2629 - 2635] Location in patent:supporting information
29943-42-8
420 suppliers
$5.00/1g

4295-99-2
166 suppliers
inquiry

![Acetonitrile, 2-[(2-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/243661-09-8.gif)