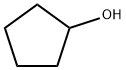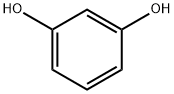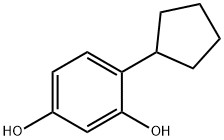
4-cyclopentylbenzene-1,3-diol synthesis
- Product Name:4-cyclopentylbenzene-1,3-diol
- CAS Number:21713-03-1
- Molecular formula:C11H14O2
- Molecular Weight:178.23
Yield:21713-03-1 35%
Reaction Conditions:
with phosphoric acid in water at 95 - 120; for 6 - 28.5 h;
Steps:
I; III EXAMPLE I The following example describes one method for preparing 4-cyclopentyl resorcinol monohydrate, as the Form I polymorph.
A round bottom flask equipped with stirrer bar was charged with resorcinol (150 g, 1.36 moles), cyclopentanol (125 ml, 1.38 moles) and phosphoric acid (85 % in water) 500 ml. The flask was fitted with a reflux condenser, purged with nitrogen and the mixture heated at 120 C (oil bath temperature) for 26 H. After this time, TLC analysis indicated that starting resorcinol was still present. Further cyclopentanol (25 ml, 0.28 moles) was added to the reaction mixture and heating continued for 2.5 hours. On cooling, the mixture was diluted with water (500 ML) and ethyl acetate (600 ml). The organic layer was separated, and the aqueous layer extracted with ethyl acetate (3 x 500 ml). The combined organic layers were neutralized by careful addition of an excess of saturated aqueous sodium hydrogen carbonate solution, washed with brine (300 ml), dried (magnesium sulfate) and concentrated. The residue was dissolved in toluene (500 ml) and water (20 ml, 1.11 moles, 0.8 eq) added. The solution was stirred for ca. 30 s and cooled in an ice/water batch with periodic stirring. After 4h the solid was filtered and left to air dry in a crystallizing dish for 16 h to give the Form I polymorph as colored crystals (118. 22g. Recrystallization in toluene afforded the Form I polymorph as white plates (93g, 35%). Found C, 67.44, H 8.22 %; C1LHL603 requires C 67.32, H 8.22 %. IR Data (MAX/CMN1) : 3199.2 br, 2963.8 s, 2863.5 s, 1624. 2 M, 1604.7 s, 1528.3 s, 1457.3 s, 1395.3 s, 1349.7 w, 1287. 4 M, 1265.2 s, 1228.0 s, 1179.4 m, 1166.9 m, 1108.1 s, 977.8 s, 826.5 s, 749.1 m, 723.9 m, 703.8 m and 627.9 m. A X-ray powder diffraction pattern was generated with a sample of 4-cyclopentyl resorcinol monohydrate produced as described above. The results of this testing are depicted in Figures I, II and Figure III (Lot No.1).
References:
WO2004/72010,2004,A1 Location in patent:Page 12-14