
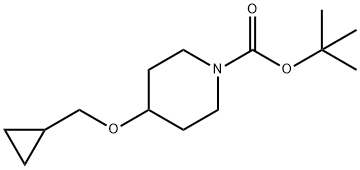
4-(CYCLOPROPYLMETHOXY)PIPERIDINE synthesis
- Product Name:4-(CYCLOPROPYLMETHOXY)PIPERIDINE
- CAS Number:865106-51-0
- Molecular formula:C9H17NO
- Molecular Weight:155.24
865106-50-9
1 suppliers
inquiry

865106-51-0
23 suppliers
$88.00/50mg
Yield:865106-51-0 100%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20;
Steps:
27.2 The second step: 4-cyclopropylmethoxypiperidine (27B)
Dissolve 4-cyclopropylmethoxypiperidine-1-carboxylic acid tert-butyl ester 27A (870mg, 3.41mmol) in 20mL of dichloromethane, add 5mL of trifluoroacetic acid, and react overnight at room temperature.LC-MS monitoring showed that the reaction was complete. The reaction liquid is adjusted to alkaline with sodium carbonate aqueous solution,It was extracted 3 times with 20 mL of dichloromethane, the dichloromethane layers were combined, dried, and spin-dried to give the product 4-cyclopropylmethoxypiperidine 27B (530 mg, 100%) as a yellow oil.
References:
CN111848591,2020,A Location in patent:Paragraph 0520-0521; 0524-0525

109384-19-2
420 suppliers
$5.00/1g

865106-51-0
23 suppliers
$88.00/50mg