
4-(di([1,1'-biphenyl]-4-yl)amino)benzaldehyde synthesis
- Product Name:4-(di([1,1'-biphenyl]-4-yl)amino)benzaldehyde
- CAS Number:159946-81-3
- Molecular formula:C31H23NO
- Molecular Weight:425.52
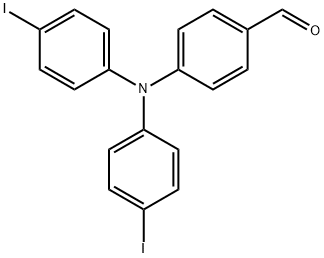
808758-81-8
56 suppliers
$59.00/250mg

98-80-6
720 suppliers
$5.00/5g
![4-(di([1,1'-biphenyl]-4-yl)amino)benzaldehyde](/CAS/20180703/GIF/159946-81-3.gif)
159946-81-3
14 suppliers
inquiry
Yield:159946-81-3 84.8%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in 1,4-dioxane;water at 80;Inert atmosphere;Suzuki Coupling;
Steps:
3.3.1. 4-(di([1,1'-biphenyl]-4-yl)amino)benzaldehyde (1)
To a round-bottomflask were added 4-(bis(4-iodophenyl)amino)benzaldehyde (1470 mg, 2.80 mmol), Pd(PPh3)4 (325 mg,0.28 mmol), K2CO3 (3100 mg, 22.4 mmol) and phenylboronic acid(821 mg, 6.72 mmol), 1,4-dioxane (32 mL) and water (8 mL), andthe mixture was stirred at 80 °C overnight. After cooling, thereaction mixture was extracted with dichloromethane. The organiclayer was dried over anhydrous Na2SO4. Afterfiltration, thesolvents were removed by rotary evaporation. The crude productwas chromatographed on silica gel eluting with petroleum etherand dichloromethane (1:1) to afford 1010 mg (84.8%) of 1 as ayellow solid.M.p.: 101.7-102.7 °C. 1H NMR (300 MHz, CDCl3) δ: 7.14 (d,J = 8.7 Hz, 2H), 7.27 (d, J = 8.7 Hz, 4H), 7.35 (t, J = 7.5 Hz, 2H), 7.45 (t,J = 7.4 Hz, 4H), 7.57-7.61 (m, 8H), 7.73 (d, J = 8.7 Hz, 2H), 9.84(s, 1H).
References:
Wang, Haobin;Bao, Benzhou;Hu, Xiangyu;Fang, Jing-Kun [Electrochimica Acta,2017,vol. 250,p. 278 - 284]
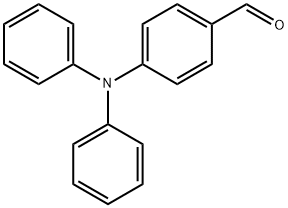
4181-05-9
201 suppliers
$14.00/1g
![4-(di([1,1'-biphenyl]-4-yl)amino)benzaldehyde](/CAS/20180703/GIF/159946-81-3.gif)
159946-81-3
14 suppliers
inquiry
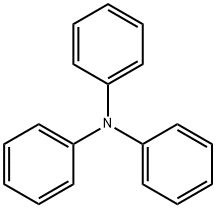
603-34-9
311 suppliers
$31.00/5g
![4-(di([1,1'-biphenyl]-4-yl)amino)benzaldehyde](/CAS/20180703/GIF/159946-81-3.gif)
159946-81-3
14 suppliers
inquiry