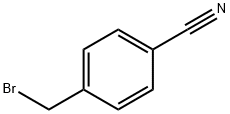
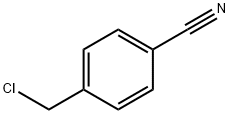
4-[(diethylamino)methyl]benzonitrile synthesis
- Product Name:4-[(diethylamino)methyl]benzonitrile
- CAS Number:69293-74-9
- Molecular formula:C12H16N2
- Molecular Weight:188.27
Yield:69293-74-9 86%
Reaction Conditions:
in tetrahydrofuran at 20; for 1.5 h;
Steps:
o-(Diethylaminomethyl)benzonitrile (13)
General procedure: Dropwise additionof o-cyanobenzylbromide (12) (10.0 g, 50 mmol) in ethanol (30mL) to cold diethylamine (57mL, 0.55 mol) was followed by 3 h of stirring at room temperature. The solution becameorange and a white precipitate was observed. Aqueous Na2CO3 (20 mL, 0.1 M) was added untilthe solution became basic to pH paper. At this time the white precipitate dissolved. Then thesolution was concentrated and extracted into an ether layer. The organic layer was washed withwater 3 times, dried over Na2SO4, and evaporated under vacuum to yield the product as a reddish-orange oil.
References:
Rajapakse, Chandima S.K.;Lisai, Maryna;Deregnaucourt, Christiane;Sinou, Véronique;Latour, Christine;Roy, Dipankar;Schrével, Joseph;Sánchez-Delgado, Roberto A. [PLoS ONE,2015,vol. 10,# 10,art. no. E0140878]