4-(DIFLUOROMETHOXY)-3,5-DIMETHOXYBENZALDEHYDE synthesis
- Product Name:4-(DIFLUOROMETHOXY)-3,5-DIMETHOXYBENZALDEHYDE
- CAS Number:446270-61-7
- Molecular formula:C10H10F2O4
- Molecular Weight:232.18
Yield:446270-61-7 74 %
Reaction Conditions:
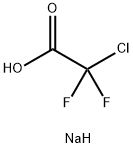
Stage #1: sodium chlorodifluoroacetate;Syringaldehydewith potassium carbonate in water;N,N-dimethyl-formamide at 100;Inert atmosphere;
Stage #2: with hydrogenchloride in water;N,N-dimethyl-formamide at 20;
Steps:
4-Difluoromethoxy-3,5-dimethoxybenzaldehyde, 2u.
The introduction of a 0-difluoromethyl substituent onto a phenol was adapted from (O'Shea et al., 2005). A mixture of 1.72 g (9.44 mmol) syringaldehyde, 2.88 g (18.88 mmol) sodium chlorodifluoroacetate and 1.57 g (11.3 mmol) K2CO3 in dimethylformamide (DMF) and H2O (17 mL+2 mL) was degassed for 5 min with N2. Then the mixture was heated to 100°C. (oil bath, preheated) for 4 h. The mixture was cooled to RT and 2.7 mL HCl 12M and 3.9 mL water were added. After stirring for 2 h 16 mL NaOH 2M were added and the mixture was diluted with Et2O and water. The layers were separated, and the aqueous layer was further extracted with Et2O (2). The combined org. layers were washed with NaOH 2M (2), water and brine, dried over Na2SO4 and concentrated in vacuo. Yield: 1.63 g (74%) product as a white solid. 1H-NMR (CDCl3): 3.95 (s, 2 MeO), 6.65 (t, 2J(H,F)=75.2 Hz, F2CH), 7.13 (s, 2 arom. H), 9.92 (s, CHO).
References:
US2022/267252,2022,A1 Location in patent:Paragraph 0027; 0110