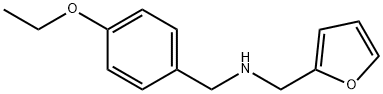
(4-ETHOXY-BENZYL)-FURAN-2-YLMETHYL-AMINE synthesis
- Product Name:(4-ETHOXY-BENZYL)-FURAN-2-YLMETHYL-AMINE
- CAS Number:436096-81-0
- Molecular formula:C14H17NO2
- Molecular Weight:231.29
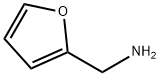
617-89-0
263 suppliers
$16.00/25g
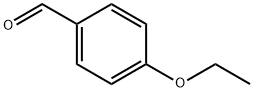
10031-82-0
275 suppliers
$6.00/5g

149-73-5
519 suppliers
$12.00/5g

436096-81-0
21 suppliers
$45.00/10mg
Yield:436096-81-0 80%
Reaction Conditions:
with sodium borohydrid;acetic acid;triethylamine in methanol;ethyl acetate;
Steps:
5.3.b 4 N-(4-ethoxybenzyl)-1-(furan-2-yl)methanamine
Example 5-3b 4 N-(4-ethoxybenzyl)-1-(furan-2-yl)methanamine 4-Ethoxy benzaldehyde (5 g, 33 mmol) and furfuryl amine (4.2 g, 43 mmoL) in a mixture of methanol (50 mL), trimethylorthoformate (10 mL) and AcOH (1 mL) were stirred at room temperature, under an atmosphere of nitrogen for 16 hours. Sodium borohydride (1.4 g, 35 mmol) was added, in 4 portions, over a period of 30 minutes (exothermic reaction). The reaction was stirred for an additional 2 hours at room temperature. The solvent was removed under vacuum and the residue taken up in ethyl acetate (150 mL). The organic phase was washed with H2O (200 mL) and aqueous phase was back extracted with ethyl acetate (2*, 100 mL). The combined organic layers were concentrated and the residue was purified on silica gel (70% ethyl acetate in hexanes with ~0.5% triethylamine) to afford N-(4-ethoxybenzyl)-1-(furan-2-yl)methanamine (6.1 g, 80%) as a clear oil. NMR (CDCl3, 400 MHz): δ 1.40 (t, J=7.2 Hz, 3H), 3.71 (s, 2H), 3.76 (s, 2H), 4.02 (q, J=7.2 Hz, 2H), 6.17 (d, J=4 Hz, 1H), 6.31 (m, 1H), 6.84 (d, J=8.8 Hz, 2H), 7.23 (d, J=8.8 Hz, 2H), 7.36 (m, 1H).
References:
US2009/274632,2009,A1

617-89-0
263 suppliers
$16.00/25g

10031-82-0
275 suppliers
$6.00/5g

436096-81-0
21 suppliers
$45.00/10mg