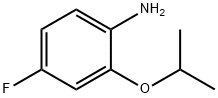
4-fluoro-2-isopropoxyaniline synthesis
- Product Name:4-fluoro-2-isopropoxyaniline
- CAS Number:148583-65-7
- Molecular formula:C9H12FNO
- Molecular Weight:169.2
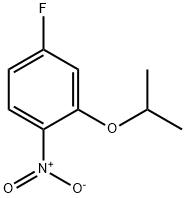
28987-46-4
22 suppliers
$45.00/100mg

148583-65-7
36 suppliers
$11.00/100mg
Yield:148583-65-7 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol;water; for 1 h;Sealed tube;Inert atmosphere;
Steps:
29 Preparation of 4-fluoro-2-iso ro ox benzenamine
A round-bottom flask was charged with 4-fluoro-2-isopropoxy-l -nitrobenzene (49.3 g, 248 mmol), EtOH (600 mL), and 10 % Pd/C (50 % in water, Degussa type E101; 10 g, 20wt% by weight of the starting nitro compound). The flask was sealed with a rubber septum, degassed, and back-filled with H2 (x3) from a balloon filled with H2. The reaction was stirred for 4 d using a H2 filled balloon. The reaction mixture was filtered through a pad of celite, and the pad of celite was rinsed with MeOH. The filtrate was evaporated to dryness to provide 4-fluoro-2-isopropoxybenzenamine (41.0 g, 98%) as a brown liquid that was used without further purification. 1H NMR (300 MHz; 6-DMSO) δ 6.67-6.72 (dd, 1H, J = 2.7Hz, 11Hz), 6.55-6.60 (dd, 1H, J = 6.0Hz, 8.7Hz), 6.43-6.49 (td, 1H, J = 2.7Hz, 8.4Hz), 4.47-4.55 (m, 3H), 1.23-1.25 (d, 6H, J = 6.0Hz); 19F NMR (282 MHz; 6-DMSO) δ - 127.4 (sextet); m/z = 170 (M+H)+.
References:
WO2013/152198,2013,A1 Location in patent:Paragraph 00588
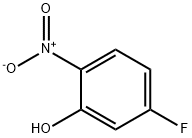
446-36-6
255 suppliers
$9.00/10g

148583-65-7
36 suppliers
$11.00/100mg