
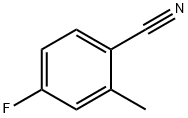
4-Fluoro-2-methylbenzoic acid synthesis
- Product Name:4-Fluoro-2-methylbenzoic acid
- CAS Number:321-21-1
- Molecular formula:C8H7FO2
- Molecular Weight:154.14

352-70-5
256 suppliers
$12.00/25mL

75-36-5
588 suppliers
$17.92/100G

321-21-1
249 suppliers
$6.00/1g
Yield:321-21-1 98 g
Reaction Conditions:
Stage #1:m-Fluorotoluene;acetyl chloride with aluminum (III) chloride in 1,2-dichloro-ethane at -5 - 10;Inert atmosphere;
Stage #2: with sodium hypochlorite;water at 20; for 2 h;
Steps:
1-3 1.Synthesis of 4'-fluoro-2'-methylacetophenone and its isomers:
In a 2000ml four-neck reaction flask, sequentially add 110g (1mol) of m-fluorotoluene,1,2-Dichloroethane 550g, under nitrogen protection, the temperature is reduced to about 0 degrees,Add 146.3 g (1.1 mol) of anhydrous aluminum trichloride,Then start to add 86.4 g (1.1 mol) of acetyl chloride dropwise, the temperature is controlled between -5 to 10 degrees,After the dropwise addition was completed, the progress of the reaction was monitored by HPLC. When m-fluorotoluene remained below 0.5%,Stop the reaction. Pour the reaction solution slowly into about 750 grams of 5% cold hydrochloric acid water, stir and dissolve completelyLeave to separate layers, remove the upper aqueous phase and wash the organic phase again.Analysis shows thatThe product 4'-fluoro-2'-methylacetophenone content is about 78%,Isomer content is about 21%.The organic phase is directly processed without further processing. 2.Synthesis of 4-fluoro-2-methylbenzoic acid and its isomers:At room temperature, slowly add about 850 grams of sodium hypochlorite aqueous solution containing about 10% of available chlorine to the organic phase of the previous step. After stirring for two hours, adjust the pH value to 3-4 with concentrated hydrochloric acid, let stand for liquid separation, and remove the aqueous phase. Then it was washed once more with water, and the solvent was distilled off at atmospheric pressure to obtain about 150 g of crude product. 3.4 Refinement of 2-fluoro-2-methylbenzoic acid:Add 300 grams of toluene and 70 grams of ethyl acetate to the crude product of the second step, heat to reflux, after dissolving, slowly cool to 20 degrees, filter and dry,98 g of product was obtained, the total yield was 63.6%, and the purity (HPLC) was about 98.5%.
References:
Beijing Aidewang Science And Technology Co., Ltd.;Ren Yalin;Zhou Weihua CN110903182, 2020, A Location in patent:Paragraph 0003

452-63-1
342 suppliers
$11.00/25g

124-38-9
134 suppliers
$175.00/23402

321-21-1
249 suppliers
$6.00/1g

452-63-1
342 suppliers
$11.00/25g

321-21-1
249 suppliers
$6.00/1g
147754-12-9
290 suppliers
$5.00/1g

321-21-1
249 suppliers
$6.00/1g

352-70-5
256 suppliers
$12.00/25mL
76-02-8
256 suppliers
$59.70/50g

321-21-1
249 suppliers
$6.00/1g

7697-23-6
238 suppliers
$7.00/1g